Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Immune checkpoint inhibitors (ICI) are a type of cancer immunotherapy being implemented as the standard of care for an increasing number of malignancies. Due to the potent nature of immune activation from these therapies, patients experience immune related adverse events (irAEs), such as inflammatory arthritis (IA). Previously published studies of ICI-IA do not include a thorough characterization of the associated immune responses to provide potential targets to treat this adverse event. Here, we seek to identify soluble factors that are associated with ICI-induced-IA and determine how those factors associate with IA severity and persistence.
Methods: We conducted an observational study of patients diagnosed with ICI-IA (n=78). Patients were treated with ICIs targeting CTLA-4, PD-1, and/or PD-L1 alone or in combination with other agents across tumor types. Serum was obtained at initial rheumatology visit with time since ICI-IA symptom onset varying between 1.7 weeks-57 months (median: 4 months). Control serum was obtained from cancer patients treated with ICIs without any diagnosed irAEs (n=17) or diagnosed with an unrelated irAE (n=17). Multiplex Mesoscale discovery assays were performed on serum to quantify the levels of interferon-gamma (IFN-γ), interleukin (IL)-10, IL-17α, IL-1α, IL-6, IL-12p70, IL-4, tumor necrosis factor (TNF)-α, and vascular endothelial growth factor (VEGF)-A. Follow up information on ICI-IA severity and persistence was obtained for up to 71 months post initial visit. Patient data was stratified based on treatment with biologics or steroids at time of blood draw. Non-parametric Mann-Whitney U tests were performed to determine statistical significance between control and ICI-induced-IA groups and between baseline cytokine levels and IA persistence. Kruskal-Wallis test was used to determine statistical significance between ICI-IA patients separated by IA severity as determined by CDAI.
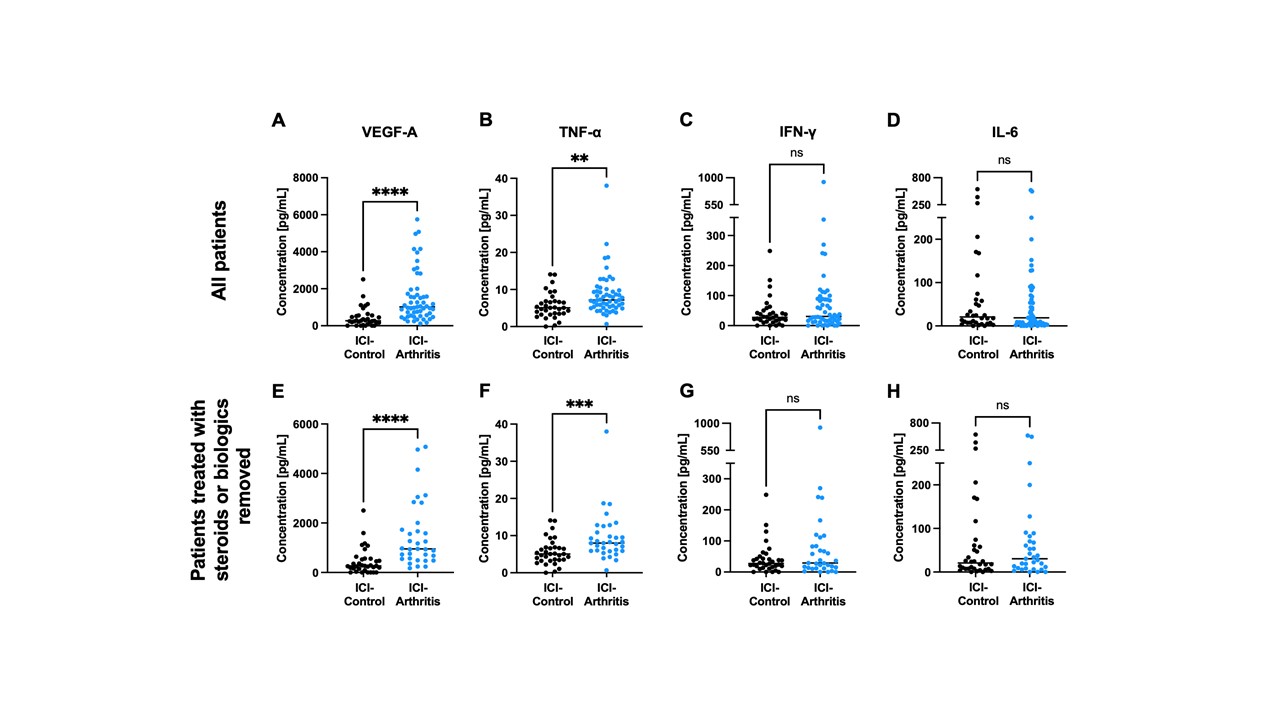
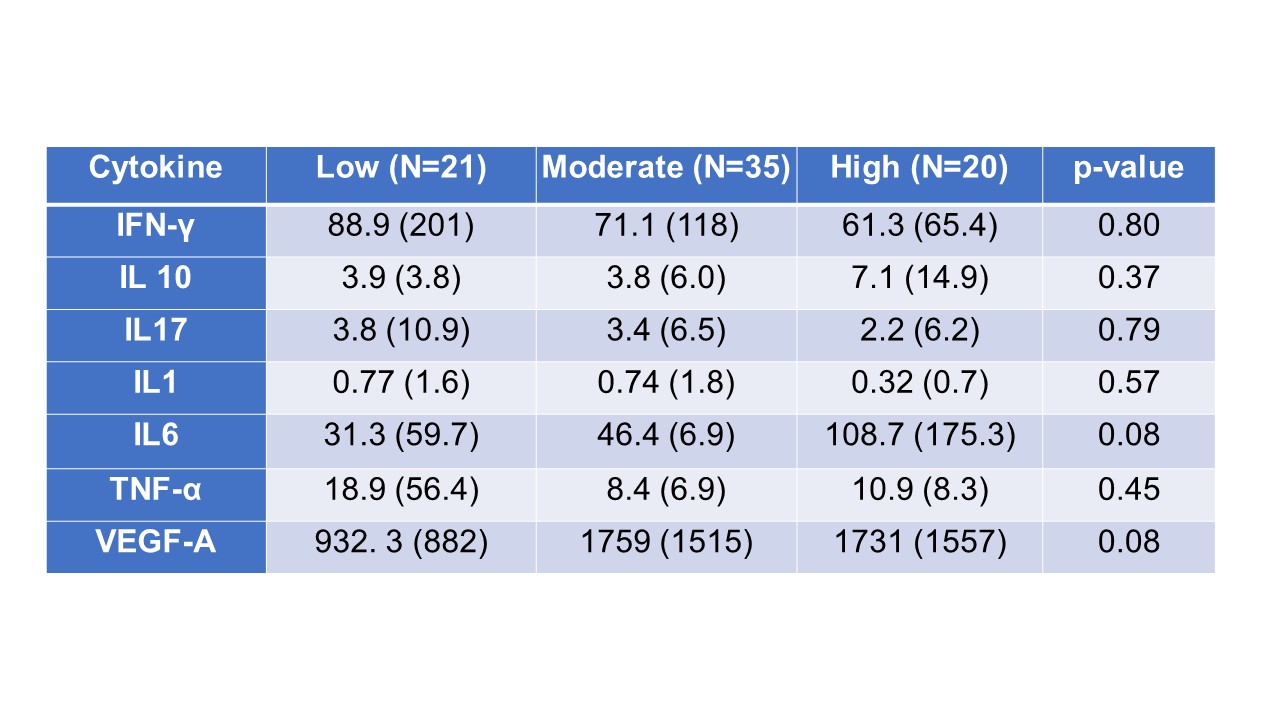
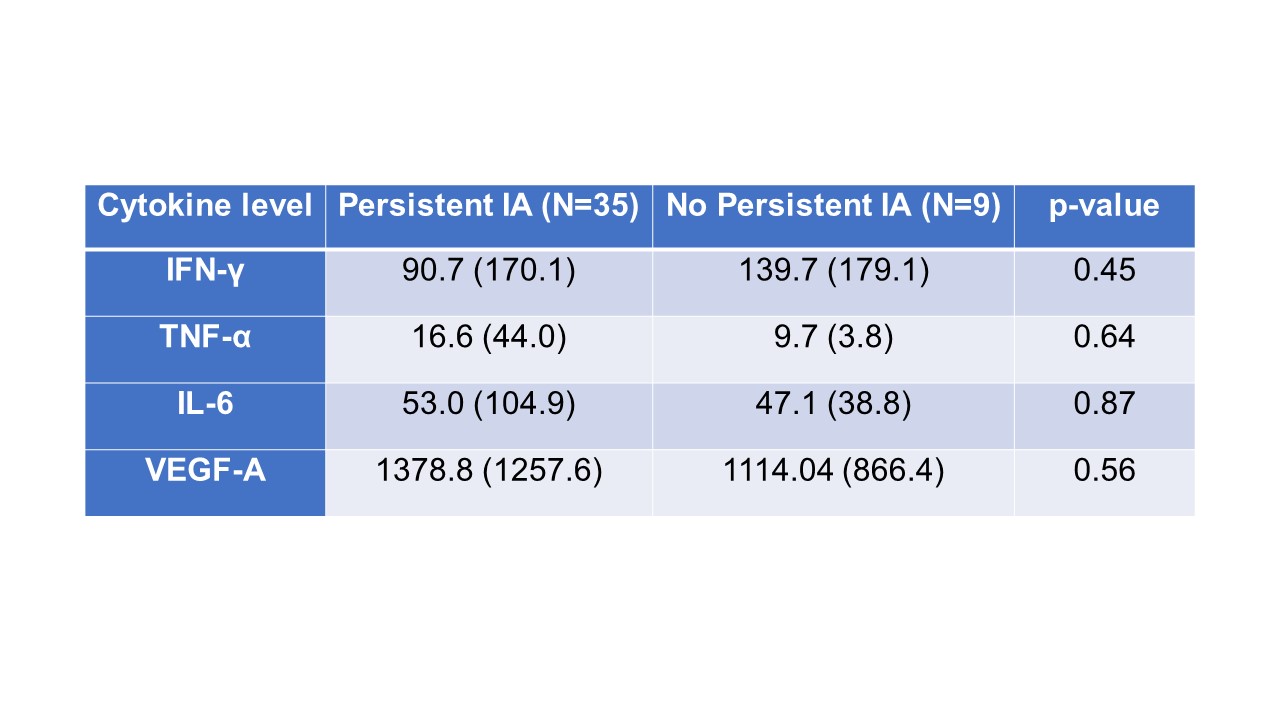
Results: VEGF-A and TNFα were both significantly elevated in the serum of patients with ICI-IA compared to ICI-control (Fig. 1A and 1B). Interestingly, IFNγ and IL-6, common inflammatory cytokines, were not significantly different (Fig. 1C and 1D). These trends were maintained when we excluded ICI-IA patients treated with steroids or biologics at the time of blood draw (Fig. 1E-H). Further, no detectable differences were observed between groups for IL-10, IL-17α, IL-1α, IL-4, and IL-12p70. We separated patients with ICI-IA by IA severity using their CDAI score; though none of the analyzed cytokines significantly correlated with severity, there were trends toward higher IL-6 and VEGF in those with higher disease activity (Table 1). Further, we separated the ICI-IA patients that were not on steroids at blood draw based on IA persistence and found no differences in baseline cytokine levels (Table 2).
Conclusion: Elevated levels of VEGF-A and TNFα are associated with ICI-IA, irrespective of steroid or biologics treatment. While neither of these factors are significantly increased with IA severity or persistence, there were trends toward higher IL-6 and VEGF and higher disease activity. These cytokines could be used as systemic biomarkers of ICI-IA and present possible treatment targets.
To cite this abstract in AMA style:
Gray-Gaillard E, Bingham C, Shah A, Cappelli L. Identification of Elevated Soluble Factors in Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis and Their Association with Severity and Persistence [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/identification-of-elevated-soluble-factors-in-immune-checkpoint-inhibitor-induced-inflammatory-arthritis-and-their-association-with-severity-and-persistence/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identification-of-elevated-soluble-factors-in-immune-checkpoint-inhibitor-induced-inflammatory-arthritis-and-their-association-with-severity-and-persistence/