Session Information
Date: Wednesday, November 15, 2023
Title: Abstracts: Miscellaneous Rheumatic & Inflammatory Diseases III
Session Type: Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Immune checkpoint inhibitors (ICIs) are revolutionizing cancer treatment. However, ICIs often lead to immune-related adverse events (irAEs). Approximately 5% of patients treated with ICIs develop arthritis (here, ICI-arthritis). ICI-arthritis not only causes joint destruction but also necessitates the discontinuation of ICI therapy. Importantly, steroids, the first-line therapy for irAEs, significantly abrogate the antitumor efficacy of ICIs. Therefore, it is critical to identify biomarkers which predict steroid resistance in irAE patients. This is particularly important in managing ICI-arthritis, because it requires long-term therapy, often with disease modifying anti-rheumatic drugs (DMARDs) in addition to systemic steroids.
Methods: Between 2019 and 2022, we enrolled 39 patients who newly developed joint pain and swelling following ICI therapy and were diagnosed with ICI-arthritis at the MD Anderson Rheumatology clinic. From the time of ICI-arthritis diagnosis, we prospectively followed these patients for one year. Simultaneously, at the time of ICI-arthritis diagnosis, we collected peripheral blood (PB) samples for serum cytokine analysis. PB samples were collected 13.4 ± 22.1 (mean ± SD) weeks after the onset of arthritis symptoms. We also collected PB samples from 11 healthy volunteers as negative controls. Levels of serum cytokines were measured by a multiplex assay. Mean differences were determined using unpaired t-test for two groups or one-way ANOVA test for three groups.
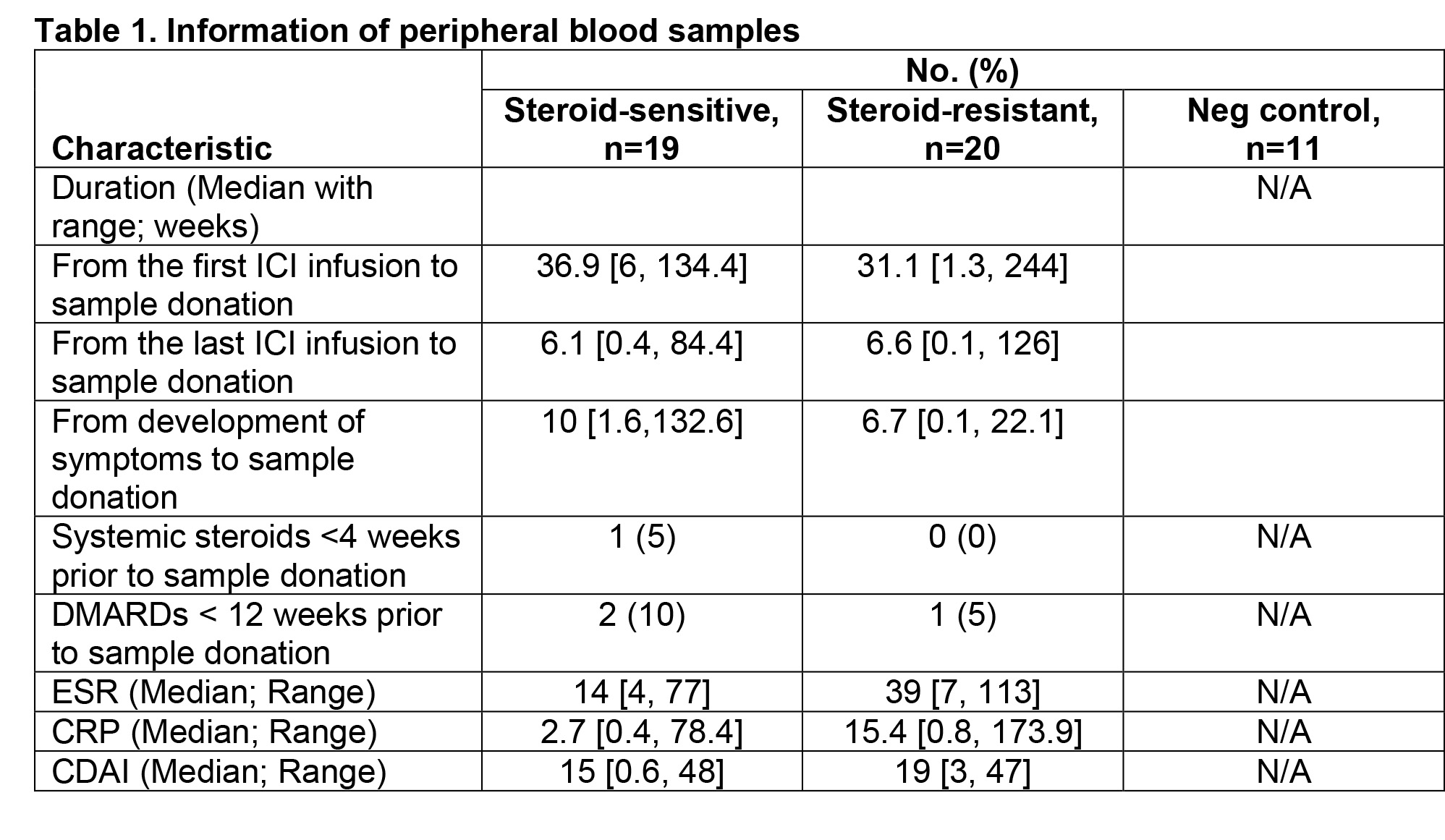
Results: Nineteen patients responded well to steroid monotherapy (here, steroid sensitive [SS] group) while twenty patients required DMARDs during the one-year followup (here, steroid resistant [SR] group). In both groups, ICI-arthritis developed primarily with PD-1 inhibitor monotherapy (74% in the SS group vs. 65% in the SR group). Fifteen patients in the SS group and 18 patients in the SR group exhibited RA-like polyarthritis. In the SR group, DMARDs were initiated 3.5 ± 3.0 months after the onset of arthritis symptoms. Hydroxychloroquine was the most commonly prescribed DMARD, followed by tocilizumab, methotrexate, and sulfasalazine. Compared to the SS group, patients in the SR group required higher doses of steroids (cumulative dose of prednisone: 1,921 ± 1,382 mg in the SR group vs. 613 ± 741 mg in the SS group; P=0.0008). A higher percentage of patients in the SS group tested positive for antinuclear antibody (10% in the SR group vs. 42% in the SS group). Characteristics of the samples were comparable between the SS and SR groups (Table 1). Serum analyses revealed that the level of interleukin (IL)-10 was higher in the SR group (14.5 ± 3.4 pg/mL in the SR group vs. 10.3 ± 5.6 pg/mL in the SS group; P=0.03). The level of serum IL-17A was higher in patients with SR arthritis, but the difference did not reach statistical significance (11.0 ± 10.9 pg/mL in the SR group vs. 5.0 ± 6.9 pg/mL in the SS group; P=0.14).
Conclusion: Th17 cells might play an important role in steroid resistance in ICI arthritis while Treg signatures might be enhanced as a compensatory mechanism. In-depth cellular, molecular, and immunologic analyses of Th17 cells will enable us to identify a biomarker predicting steroid resistance in ICI arthritis.
To cite this abstract in AMA style:
Thimmapuram R, Buni M, Lu H, Tayar J, Li Y, Suarez-Almazor M, Rico R, Turner N, Nurieva R, Kim S. Identification of Biomarkers Predicting Steroid Resistance in Immune Checkpoint Inhibitor-Induced Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/identification-of-biomarkers-predicting-steroid-resistance-in-immune-checkpoint-inhibitor-induced-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identification-of-biomarkers-predicting-steroid-resistance-in-immune-checkpoint-inhibitor-induced-arthritis/