Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: We have shown that
repeated immunization with antigen causes systemic autoimmunity in mice
otherwise not prone to spontaneous autoimmune diseases. Overstimulation of CD4
T cells led to the development of autoantibody-inducing CD4 T (aiCD4 T) cell which had
undergone de
novo T
cell receptor (TCR) revision. These cells originated predominantly from
thymus-passed lymphocytes at the periphery. These aiCD4 T cells were capable of inducing
a variety of autoantibodies and stimulating CD8 T cells, driving them to become
antigen-specific cytotoxic T lymphocytes (CTL). These CTLs could be further
matured thru antigen cross-presentation, after which they caused tissue injury
identical to that observed in SLE. Thus, systemic autoimmunity appears to be
the inevitable consequence of over-stimulating the host’s immune system by
repeated immunization with antigen to levels that surpass system’s
self-organized criticality. We have thus proposed the �eSelf-organized
criticality theory’ of autoimmunity (Tsumiyama K et al. PLoS ONE 4(12): e8382,
2009), explaining the cause of SLE. Here we identify the aiCD4 T cell responsible
for inducing SLE as the DOCK8+ CD4 T cell subset.
Methods: BALB/c mice were
immunized 12x with OVA. Isolated CD4 subsets were adoptively transferred into
naïve recipients, and aiCD4 T cells were screened
by analysis of the autoantibodies generated in the sera of recipient mice.
Membrane fractions of the CD4 T cell subsets responsible for inducing the
disease were isolated and examined by mass spectrometry, and candidate proteins
specifically expressed in the CD45RBloCD122lo CD4 T cell fraction were
identified by antibody staining. The CD4 T subset expressing the candidate
protein of interest was confirmed to be the aiCD4 T cell subset by cell transfer
into naïve recipients.
Results: Thru fractionation of CD4
T cell subsets and microarray analyses, we found that aiCD4 T cells belonged to
CD45RBloCD122loPD1+ CD4 subset. We then
isolated membrane and cytoplasmic fractions of these cells and analyzed their
proteins by mass spectrometry, and found that among several candidate proteins,
DOCK8 was specifically expressed in the membrane fraction of this subset.
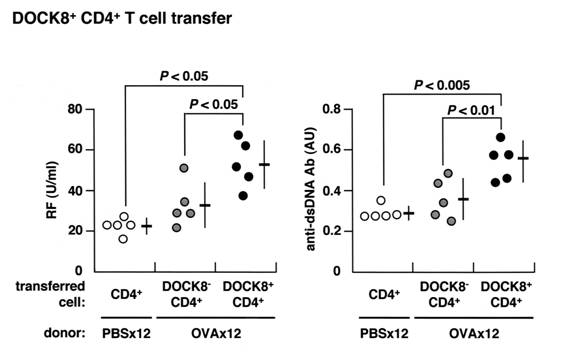
Adoptive transfer of DOCK8+ CD4 cells from mice immunized 12x with OVA caused
an increase in both RF and anti-dsDNA antibody production in the naïve
recipients.
Conclusion: The aiCD4 T cell subset that
induces SLE is identified as DOCK8+ CD4 T cells.
To cite this abstract in AMA style:
Shiozawa S, Miyazaki Y, Shiozawa K, Tsumiyama K. Identification of Autoantibody-Inducing CD4 T Cell (aiCD4 T cell) That Causes Systemic Lupus Erythematosus (SLE) As DOCK8+ CD4 T Cell: Proof of Concept of Self-Organized Criticality Theory [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/identification-of-autoantibody-inducing-cd4-t-cell-aicd4-t-cell-that-causes-systemic-lupus-erythematosus-sle-as-dock8-cd4-t-cell-proof-of-concept-of-self-organized-criticality-theory/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identification-of-autoantibody-inducing-cd4-t-cell-aicd4-t-cell-that-causes-systemic-lupus-erythematosus-sle-as-dock8-cd4-t-cell-proof-of-concept-of-self-organized-criticality-theory/