Session Information
Date: Sunday, November 12, 2023
Title: Abstracts: T Cell Biology & Targets in Autoimmune & Inflammatory Disease
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Chimeric Antigen Receptor Regulatory T cells (CAR-Tregs) are an emerging strategy to restore immune tolerance during auto- or allo-immune conditions. However, most autoimmune diseases do not have a single or consistent pathogenic antigen to target. OX40L is a co-stimulatory protein expressed on activated antigen presenting cells (APCs). Polymorphisms in OX40L that lead to increased expression are associated with systemic lupus erythematosus (SLE), and the frequency of OX40L+ APCs tracks with disease activity in SLE patients. OX40L may thus serve as an ideal CAR-Treg target in SLE.
Methods: We engineered a CAR construct containing a single chain variable fragment of anti-OX40L IgG (αOX40L scFv CAR) under transcriptional control of the FOXP3 promoter to constrain CAR expression to canonical Treg. CAR-Tregs were compared with polyclonal Control-Tregs, transduced with a Neon Green reporter-encoding construct, for key features, including expression of immune regulatory proteins, suppression of T cell activation, and inhibition of APC functions in vitro.
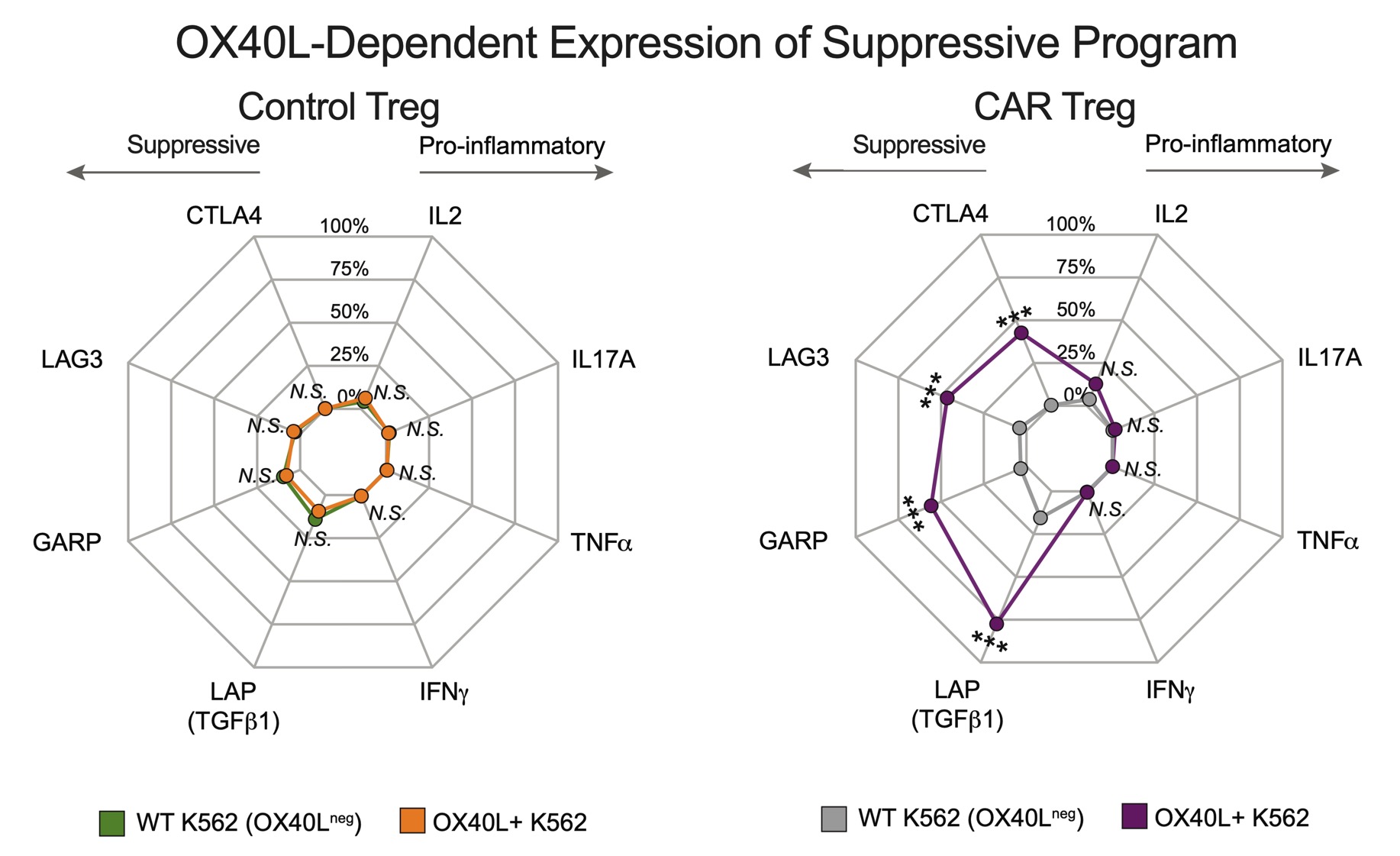
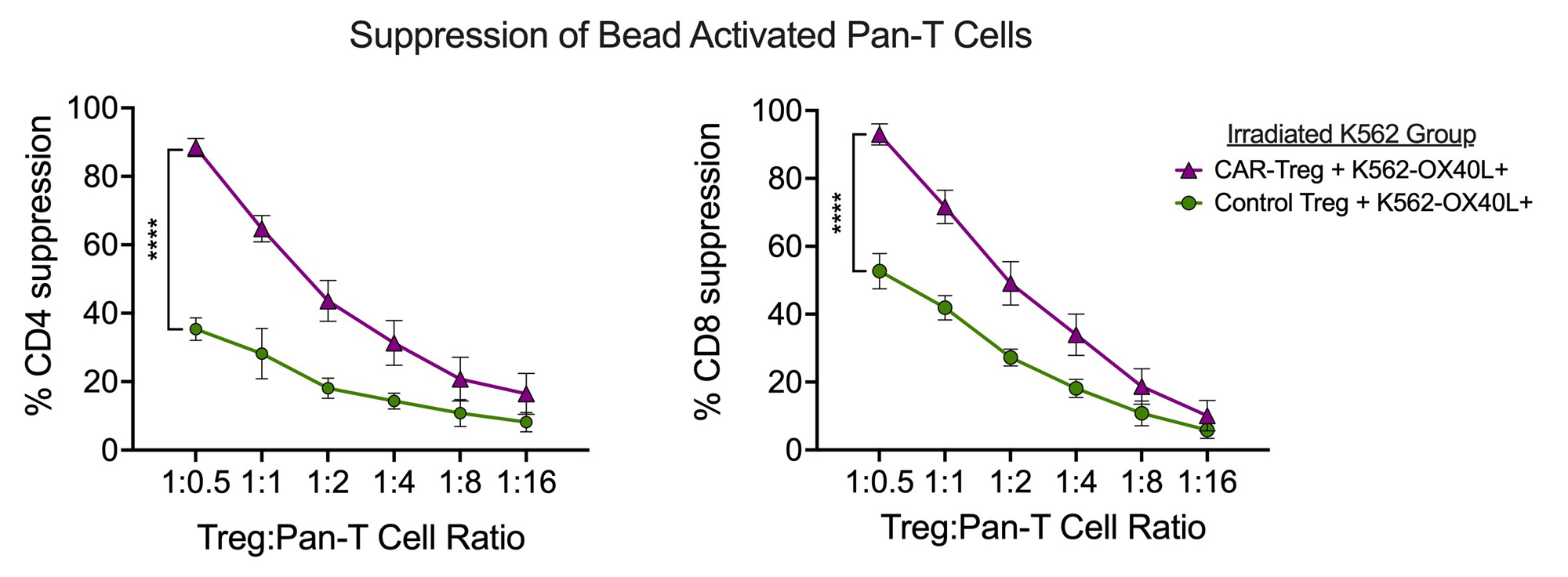
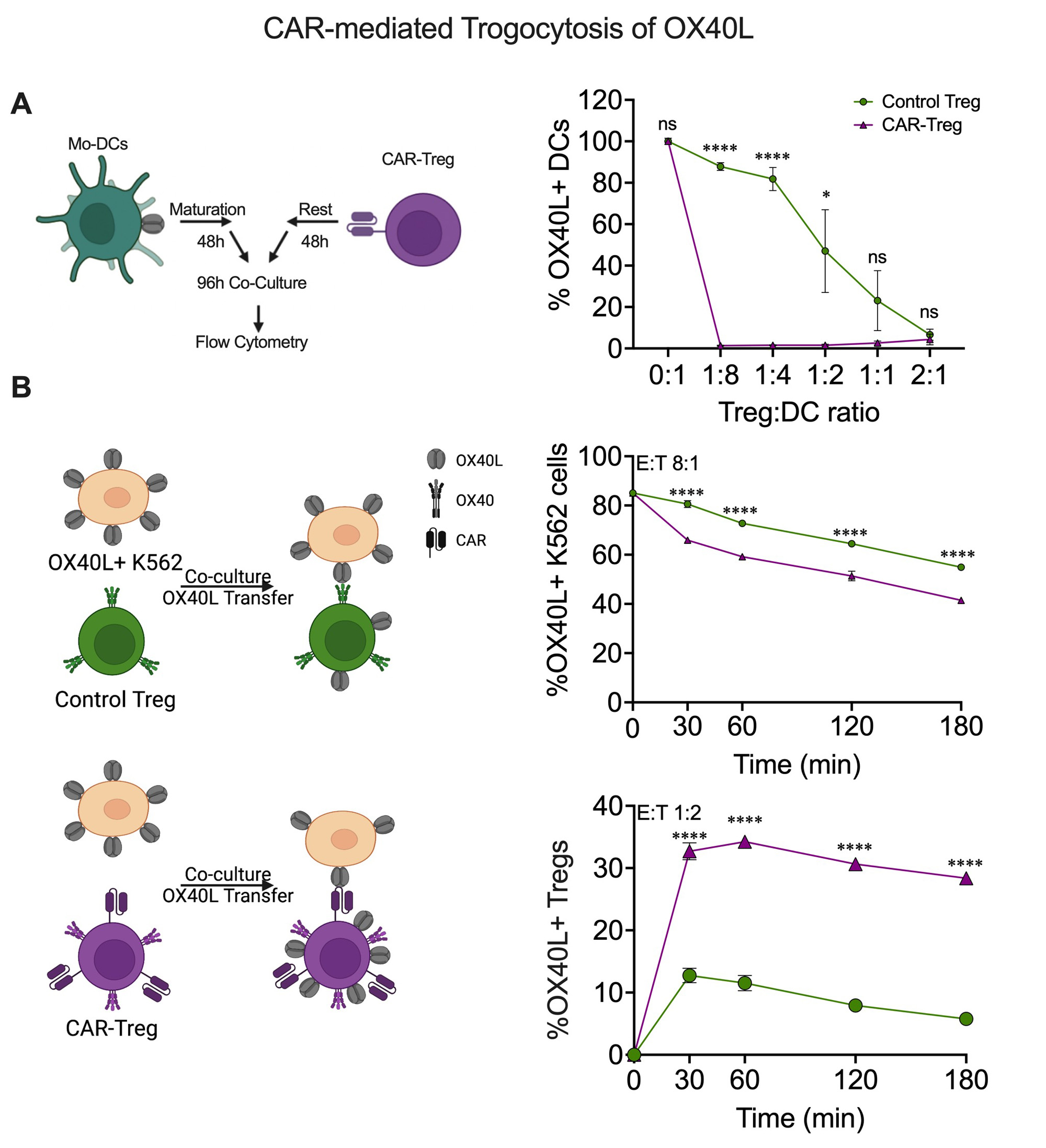
Results: αOX40L scFv CAR expression was stable and selectively expressed in FOXP3+ Tregs during in vitro expansion over three weeks. CAR-Tregs were strongly activated by OX40L+ K562 cells (Figure 1), which, relative to wild type K562 cells, drove expression of Treg-associated immune regulatory proteins LAG3 (5.6-fold), CTLA4 (14.8-fold), GARP (6.9-fold), and LAP (5.5-fold) without induction of pro-inflammatory cytokines (IL-2, TNFα, IL-17A, IFNγ). CAR-Tregs more potently suppressed anti-CD3/CD28 mAb mediated CD4+ and CD8+ T cell proliferation than Control-Tregs (maximum suppression at a 2:1 Treg:T-cell responder ratio: 88.5%±3.7% vs.35.3±4.6% for CD4+ responders, p < 0.0001) (Figure 2). CAR-Tregs also led to a greater reduction of OX40L on the surface of monocyte-derived dendritic cells (mo-DCs) relative to Control-Tregs as a result of enhanced OX40L trogocytosis – findings confirmed using OX40L+ K562 cells (Figure 3).
Conclusion: We designed a novel CAR-Treg stimulated by OX40L on activated APCs. The αOX40L scFv CAR was selectively expressed in Tregs due to control by a FOXP3 promoter, and CAR-Tregs preserved their anti-inflammatory expression profile. CAR-Tregs had superior in vitro suppression of both activated T cells and dendritic cells relative to Control-Tregs. This may be due to universal and potent activation of CAR-Tregs vs. polyclonal Control-Tregs but also from CAR mediated trogocytosis of OX40L on APCs making it unavailable for T-cell co-stimulation. Future work will be aimed at designing a mouse OX40L targeted CAR-Treg to assess in traditional murine SLE models. Overall, we demonstrate a unique approach to CAR-Treg design for treating autoimmune disorders by directing CAR-Treg against activated, disease-associated APCs.
To cite this abstract in AMA style:
Wobma H, Rui X, Alvarez-Calderon F, Gerdemann U, McGuckin C, Blazar B, Tkachev V, Kean L. Human Chimeric Antigen Receptor (CAR)-Tregs Targeting OX40L for Treatment of Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/human-chimeric-antigen-receptor-car-tregs-targeting-ox40l-for-treatment-of-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/human-chimeric-antigen-receptor-car-tregs-targeting-ox40l-for-treatment-of-systemic-lupus-erythematosus/