Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: It is unknown if steroid use for RA patients could interfere with the benefits of immune checkpoint inhibitors (ICI) as cancer therapies. Additionally, it is unknown what a “safe” daily dosage of steroids would be in ICI-treated RA patients. Prior studies have been biased by inclusion of multiple autoimmune diseases and/or multiple cancers. Therefore, we examined the association between steroid use after ICI initiation and overall survival (OS) specifically among RA patients with metastatic non-small cell lung cancer (mNSCLC).
Methods: Medicare fee for service claims data (01/2006 to 12/2019) consisting of a 100% sample of patients with RA was used. Patients included were ≥66 years of age and had an RA diagnosis prior to a mNSCLC diagnosis. RA was defined as having two RA diagnosis claims (ICD-9/10-CM codes) at least two months apart plus DMARD treatment. NSCLC was defined as having an (ICD-9/10) diagnosis code for malignant neoplasm of lung and bronchus. Patients had to have initiated nivolumab, pembrolizumab or atezolizumab between 2015-2019, when it was approved only for metastatic NSCLC. Mean daily prednisone use was calculated in the 91 days after ICI initiation and categorized as none, ≤10mg, >10mg. Landmark Kaplan Meier (KM) curves and adjusted Cox Proportional Hazard models were created using time from ICI initiation to death, for OS. Steroids, DMARDS, and opioids were categorized in the 91 days prior to ICI initiation and in the following 91 days after. Patients had to survive 91 days after ICI initiation to be included in the landmark analysis.
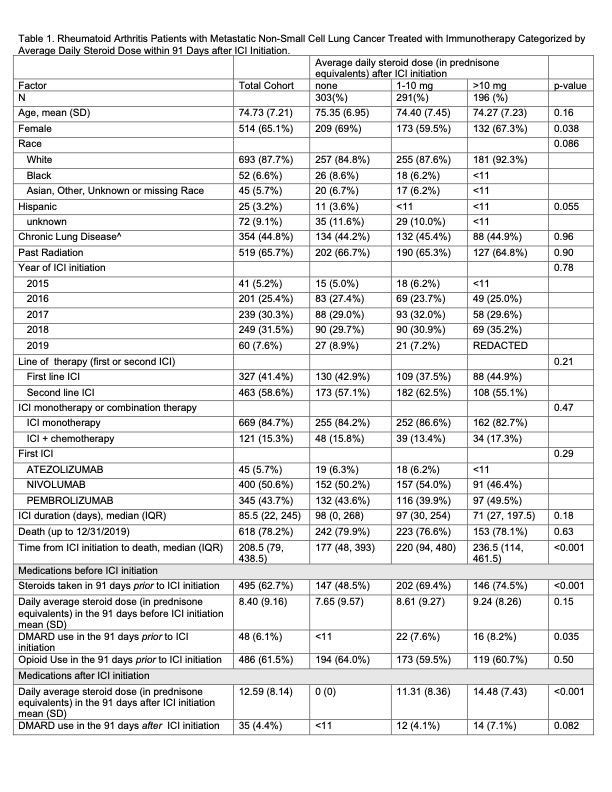
Results: We identified 790 eligible patients, mean age was 74 years (SD 7.2), 65.1% (N=514) female, 87.7% (N=693) White, and 3.2% (N=25) Hispanic. Most, 58.6% (N=463) used ICI as second-line therapy (e.g after chemotherapy). A majority 84.7% used ICI as monotherapy rather than in combination with chemotherapy. Opioids were prescribed to 61.5% of patients prior to ICI initiation. In the 91 days following ICI initiation, 38.4% (N=303) took no steroids, 36.8% (N=291) took ≤10mg daily, and 24.8% (N=196) took >10mg. Only 6.1% of patients were on DMARDs in the 91 days prior to ICI initiation (mostly methotrexate) and 4.4% were on DMARDs in the 91 days after ICI initiation. The large majority of DMARD treated patients were on concomitant steroids (Table 1).
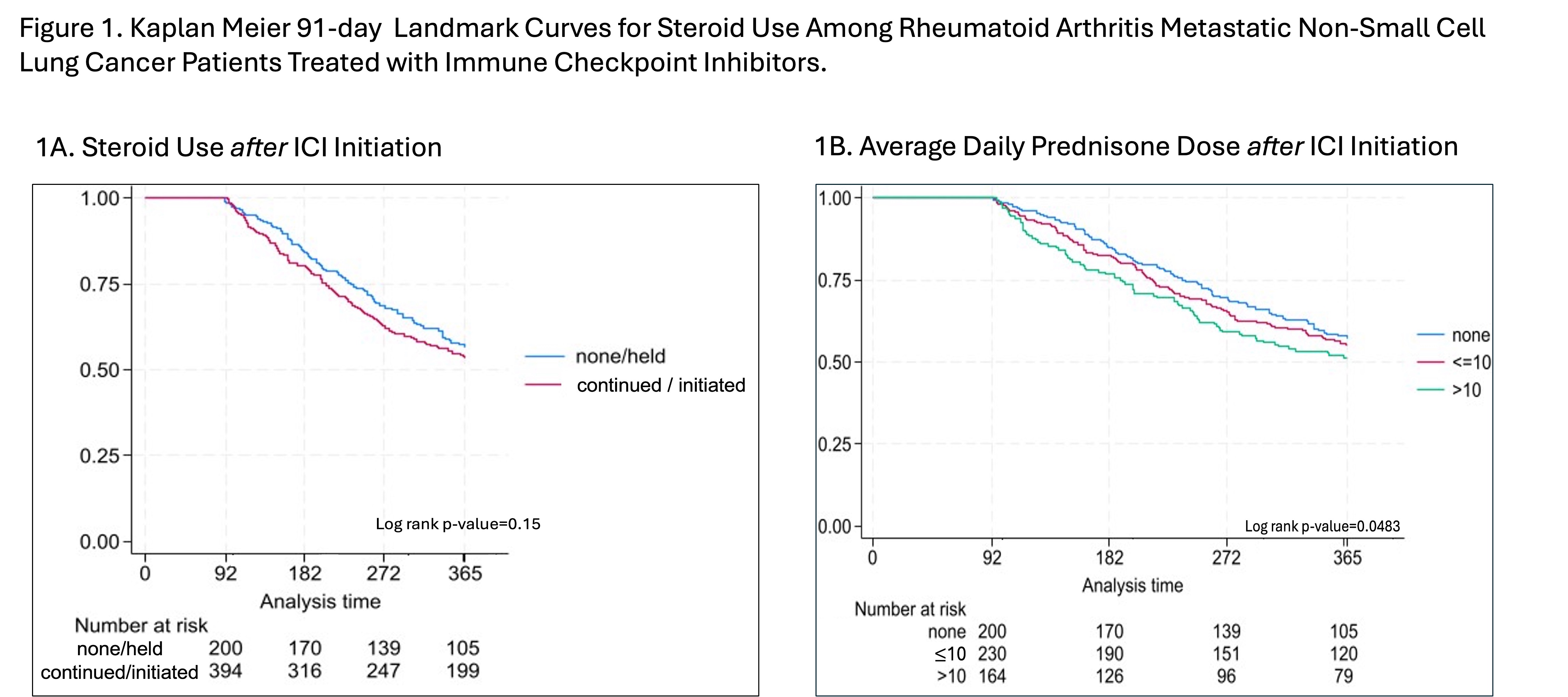
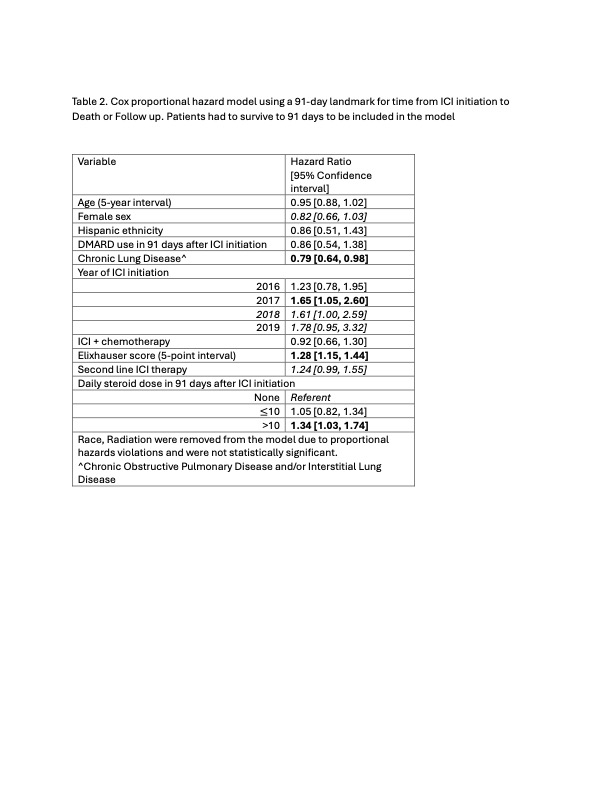
Figure 1A compares OS in patients who did versus did not take steroids in the first 91 days after ICI initiation (p=0.15), while Figure 1B demonstrates a dose response relationship between post ICI steroid use and OS (p=0.048). In adjusted models, patients using >10mg steroids had worse OS (HR 1.34, 95%CI [1.03, 1.74]) compared to patients not taking steroids after ICI initiation. There was no significant difference between the ≤10mg and no steroid groups (HR, 1.05 95%CI [0.82, 1.34]) (Table 2).
Conclusion: We found that higher doses of steroids ( >10mg average daily dose) taken in the first 91 days after ICI initiation were associated with worse OS among ICI-treated RA patients with mNSCLC whereas low doses of steroids (≤10mg average daily dose) were not associated with a reduction in OS. Few patients were utilizing DMARDs either immediately before or after ICI initiation.
To cite this abstract in AMA style:
Jannat-Khah D, Curtis J, Xie F, Saxena A, Bass A. Higher Oral Steroid Dose Is Associated with Worse Survival in Immune Checkpoint Inhibitor-Treated Rheumatoid Arthritis Patients with Metastatic Non-Small Cell Lung Cancer [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/higher-oral-steroid-dose-is-associated-with-worse-survival-in-immune-checkpoint-inhibitor-treated-rheumatoid-arthritis-patients-with-metastatic-non-small-cell-lung-cancer/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/higher-oral-steroid-dose-is-associated-with-worse-survival-in-immune-checkpoint-inhibitor-treated-rheumatoid-arthritis-patients-with-metastatic-non-small-cell-lung-cancer/