Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Although immune checkpoint inhibitors (ICI) have transformed cancer treatment, they unleash a range of immune-related adverse events (irAE), including inflammatory arthritis (ICI-IA). Glucocorticoids are often used to treat irAEs, but there is concern that this may negatively impact cancer survival, particularly when used at higher doses. In this study, we carefully tracked glucocorticoid use and cumulative exposure in patients initiating treatment for ICI-IA, with the goal of quantifying its relationship to progression-free survival (PFS).
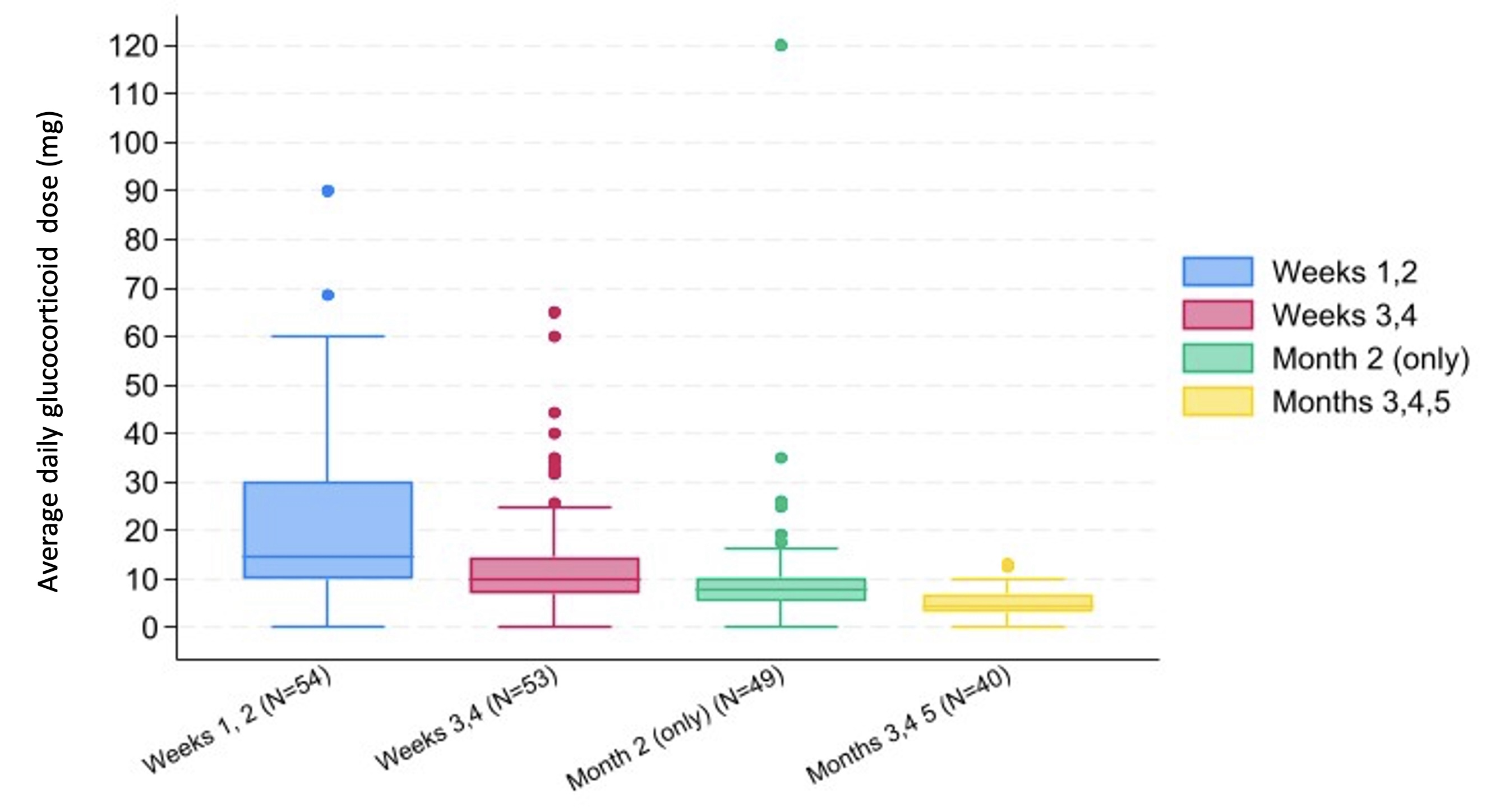
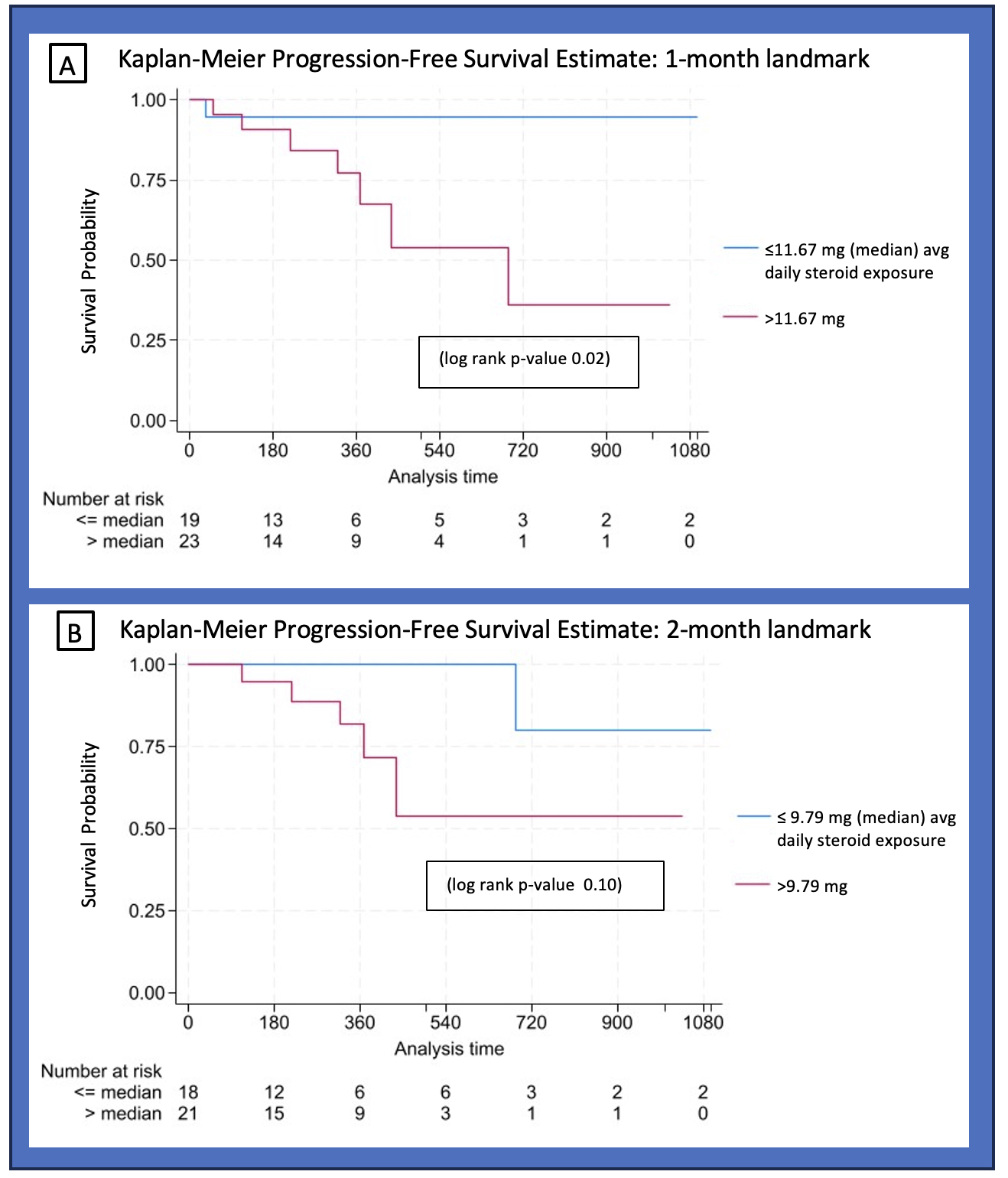
Methods: We included patients from a single center enrolled since February 2023 in a prospective rheumatic irAE registry (RADIOS) who were treated with glucocorticoids for ICI-IA. ICI-IA was defined as inflammatory arthritis, polymyalgia rheumatica, or inflammatory arthralgia. Patients started on glucocorticoids for other irAE and patients with preexisting autoimmune disease were excluded. Demographics, cancer type, ICI type, DMARD use and glucocorticoid treatment data were collected. Cumulative glucocorticoid exposure and average daily dose were recorded at specific intervals: first 2 weeks after glucocorticoid initiation, second 2 weeks, month 2, and months 3, 4, 5. Comparisons between patients with above vs below the median average daily glucocorticoid dose in the first month after glucocorticoid initiation were calculated using Fisher’s exact, Wilcoxon rank sum, and T-tests as appropriate. PFS, measured from glucocorticoid initiation to documented radiographic cancer progression or death, was visualized using Kaplan-Meier curves with a 1-month and 2-month landmark, and a log-rank test was calculated. Patients whose cancer progressed or who died prior to the landmark were excluded from the analysis. Patients were followed through 4/30/2024.
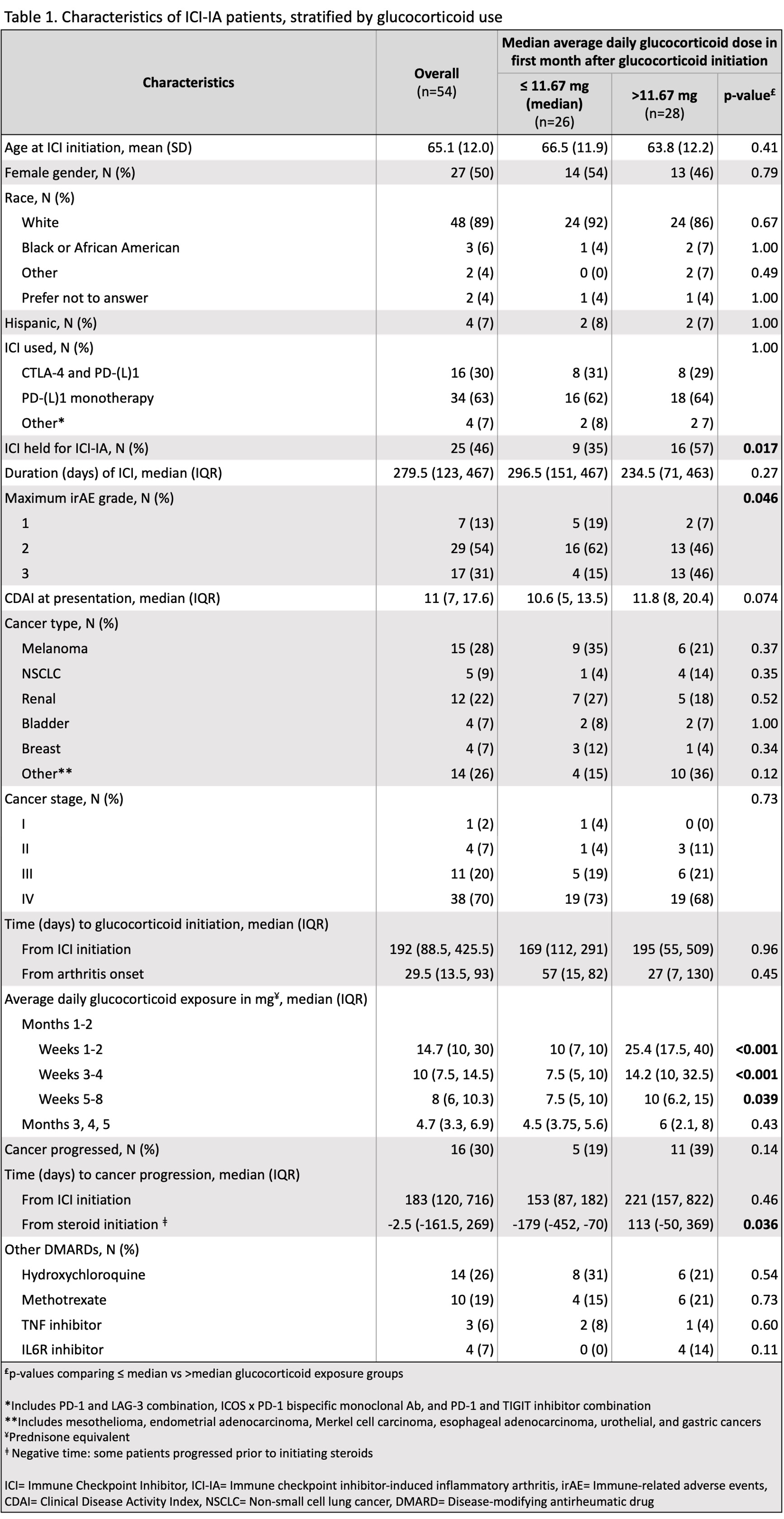
Results: Fifty-four patients with melanoma, renal cell carcinoma, non-small cell lung cancer (NSCLC), bladder, breast, and other cancers were included, and 70% had stage IV cancer at ICI initiation. Mean (SD) age at ICI initiation was 65.1 (12.0); 50% of patients were female and 89% were White. Glucocorticoid exposure for the aforementioned time frames is shown in Figure 1. Patients with above median cumulative glucocorticoid exposure ( >11.67 mg average daily dose) in their first month were more likely to have higher grade ICI-IA (46% vs 15% grade 3, 46% vs 62% grade 2, 7% vs 19% grade 1, p=0.046) and to have their ICI held (57% vs 35%, p=0.017) (Table 1). Time from ICI initiation to steroid initiation, cancer type, stage, and ICI used were not statistically different between the two groups. In a 1-month landmark analysis, patients with above median steroid exposure had worse progression-free survival (log-rank p=0.02) while differences in the 2-month landmark analysis were not significant (Figure 2).
Conclusion: Higher cumulative glucocorticoid exposure in the first month of ICI-IA treatment is associated with worse progression-free survival. Time from ICI initiation to steroid initiation was not different between patients with above vs below median steroid exposure; however, patients on higher dose glucocorticoids were more likely to have their ICI held.
To cite this abstract in AMA style:
Nong M, Jannat-Khah D, Chan K, Ghosh N, Bass A. Higher Early Cumulative Glucocorticoid Exposure Is Associated with Worse Progression-Free Survival in Patients with Immune Checkpoint Inhibitor Inflammatory Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/higher-early-cumulative-glucocorticoid-exposure-is-associated-with-worse-progression-free-survival-in-patients-with-immune-checkpoint-inhibitor-inflammatory-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/higher-early-cumulative-glucocorticoid-exposure-is-associated-with-worse-progression-free-survival-in-patients-with-immune-checkpoint-inhibitor-inflammatory-arthritis/