Session Information
Date: Sunday, November 7, 2021
Title: Epidemiology & Public Health Poster II: Inflammatory Arthritis – RA, SpA, & Gout (0560–0593)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Older people are often underrepresented in trials because the generally high number of comorbid conditions (1). The objective of this abstract is to document the baseline status and frequency of comorbid conditions and concomitant medications in the GLORIA study population which consists of older rheumatoid arthritis (RA) patients.
Methods: This double-blind, randomized, placebo-controlled, multicenter trial (2) was open for patients with RA according to the 1987 or 2010 criteria, age ≥65 years, and disease activity score of 28 joints (DAS28) of ≥2.6. Its pragmatic design featured minimal exclusion criteria tailored to seniors. Patients were recruited from rheumatology clinics in 7 European countries. Eligible patients were randomized to two years of treatment with daily 5 mg prednisolone or matching placebo. All other medication was allowed, except for glucocorticoids.
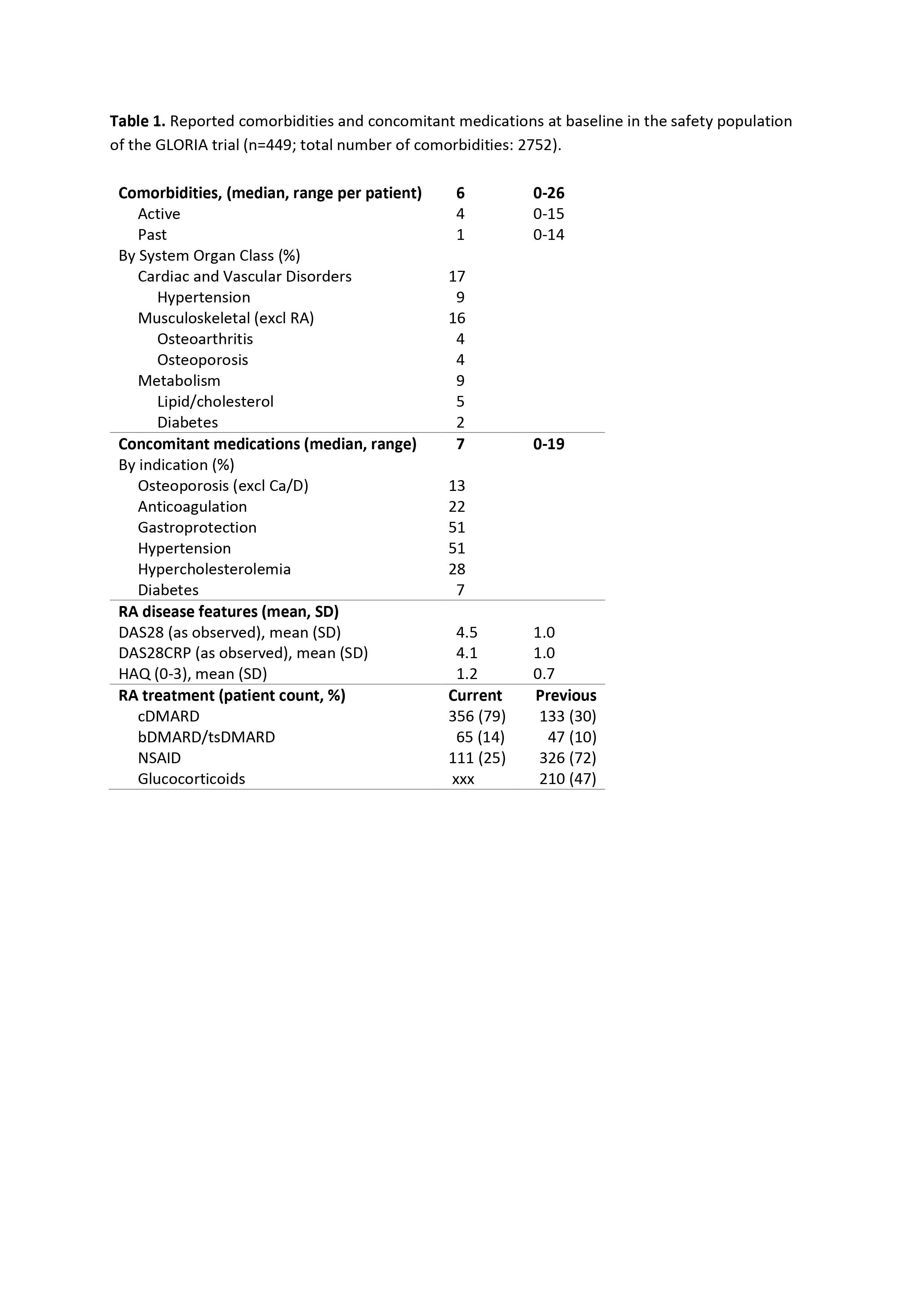
Results: The population consists of 451 patients with mean disease duration 10.6 (Q1-Q3: 3-15) years. The majority (70%) is female, mean age is 72.5 (Q1-Q3: 68-76, range: 65-88) years, 66% were positive for rheumatoid factor and 56% for ACPA. Patients had a median of 6 comorbidities besides RA (4 active) and therefore used multiple medications (median 7, max 19 for all indications) (Table 1). The most common comorbidities in this older population are, by system organ class: cardiac and vascular disorders (17%), musculoskeletal and connective tissue disorders excl. RA (16%), and metabolism and nutrition disorders (9%). At baseline, patients were most frequently on medication for gastroprotection or hypertension (both 51%). Most patients also have an extensive history of anti-rheumatic treatment. At the start of the trial most patients (79%) were on cDMARD treatment; 14% were on bDMARDs/tsDMARDs. Almost half of the patients previously had been treated with glucocorticoids, with a mean duration of 3.4 years and a mean last dose of 4.6 mg/day.
Conclusion: Our data show the medical reality of a study population aged 65+ when minimal eligibility criteria are applied. These patients with multiple comorbidities next to RA and concomitant treatment are similar to patients seen in routine care. This increases generalizability and relevance of the trial results. At the same time, interpretation of those results, especially those regarding safety of the intervention, will need to take comorbidity into account.
References
1. Palmowski A, Buttgereit T, Palmowski Y, Nielsen SM, Boers M, Christensen R, et al. Applicability of trials in rheumatoid arthritis and osteoarthritis: A systematic review and meta-analysis of trial populations showing adequate proportion of women, but underrepresentation of elderly people. Semin Arthritis Rheum. 2019;48(6):983-9.
2. Hartman L, Rasch LA, Klausch T, Bijlsma JWJ, Christensen R, Smulders YM, et al. Harm, benefit and costs associated with low-dose glucocorticoids added to the treatment strategies for rheumatoid arthritis in elderly patients (GLORIA trial): study protocol for a randomised controlled trial. Trials. 2018;19(1):67.
This trial was funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 634886.
To cite this abstract in AMA style:
Boers M, Hartman L, Opris-Belinski D, Bos R, Kok M, Pereira da Silva J, Griep E, Klaasen R, Allaart C, Baudoin P, Raterman H, Szekanecz Z, Buttgereit F, Masaryk P, Klausch L, Paolino S, Schilder A, Lems W, Cutolo M. High Number of Comorbidities and Concomitant Medications at Baseline in the Glucocorticoid Low-dose Outcome in Rheumatoid Arthritis (GLORIA) Study: An Older Population with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/high-number-of-comorbidities-and-concomitant-medications-at-baseline-in-the-glucocorticoid-low-dose-outcome-in-rheumatoid-arthritis-gloria-study-an-older-population-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/high-number-of-comorbidities-and-concomitant-medications-at-baseline-in-the-glucocorticoid-low-dose-outcome-in-rheumatoid-arthritis-gloria-study-an-older-population-with-rheumatoid-arthritis/