Session Information
Date: Wednesday, November 15, 2023
Title: Abstracts: RA – Diagnosis, Manifestations, and Outcomes IV: Pre-RA & RA Diagnosis
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: We attempted to replicate and expand previous findings of an increased abundance of Prevotellaceae in early untreated Rheumatoid Arthritis (RA) or its preclinical stages, in first-degree relatives of RA patients (FDRs).1
Methods: FDRs from the SCREEN-RA cohort (Evaluation of a SCREENing strategy for RA)2 were categorized in four groups:
- Controls: healthy asymptomatic FDRs
- High genetic risk: asymptomatic FDRs with two shared epitope (SE) copies
- Autoimmunity: asymptomatic FDRs with RA-associated autoimmunity
- Symptomatic: clinically suspect arthralgias (CSA) or untreated new-onset RA
Fecal samples were collected and immediately frozen. DNA was extracted and V3-V4 16S regions were sequenced on a MiSeq platform. Output was processed with DADA2 pipeline on R, using Silva 138v database. Cells counts (cytometry) and calprotectin (ELISA) were obtained for each fecal sample. Microbial community composition and differential analysis were conducted using non-parametric tests, such as PERMANOVA, Wilcoxon and Kruskal-Wallis, or Aldex2 R-package to account for data compositionality.
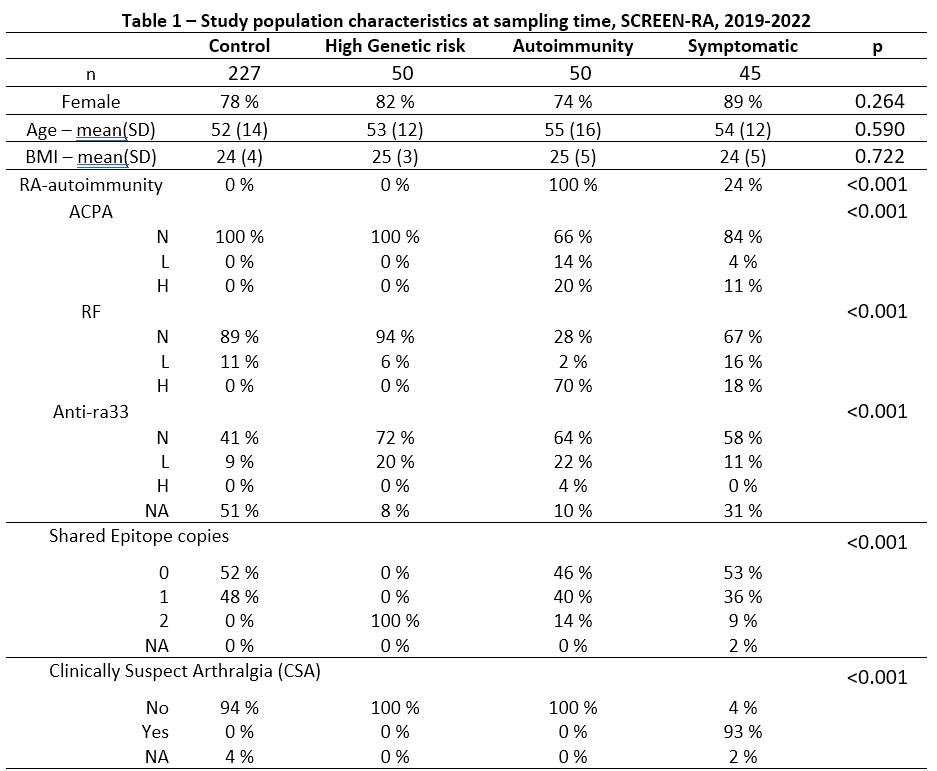
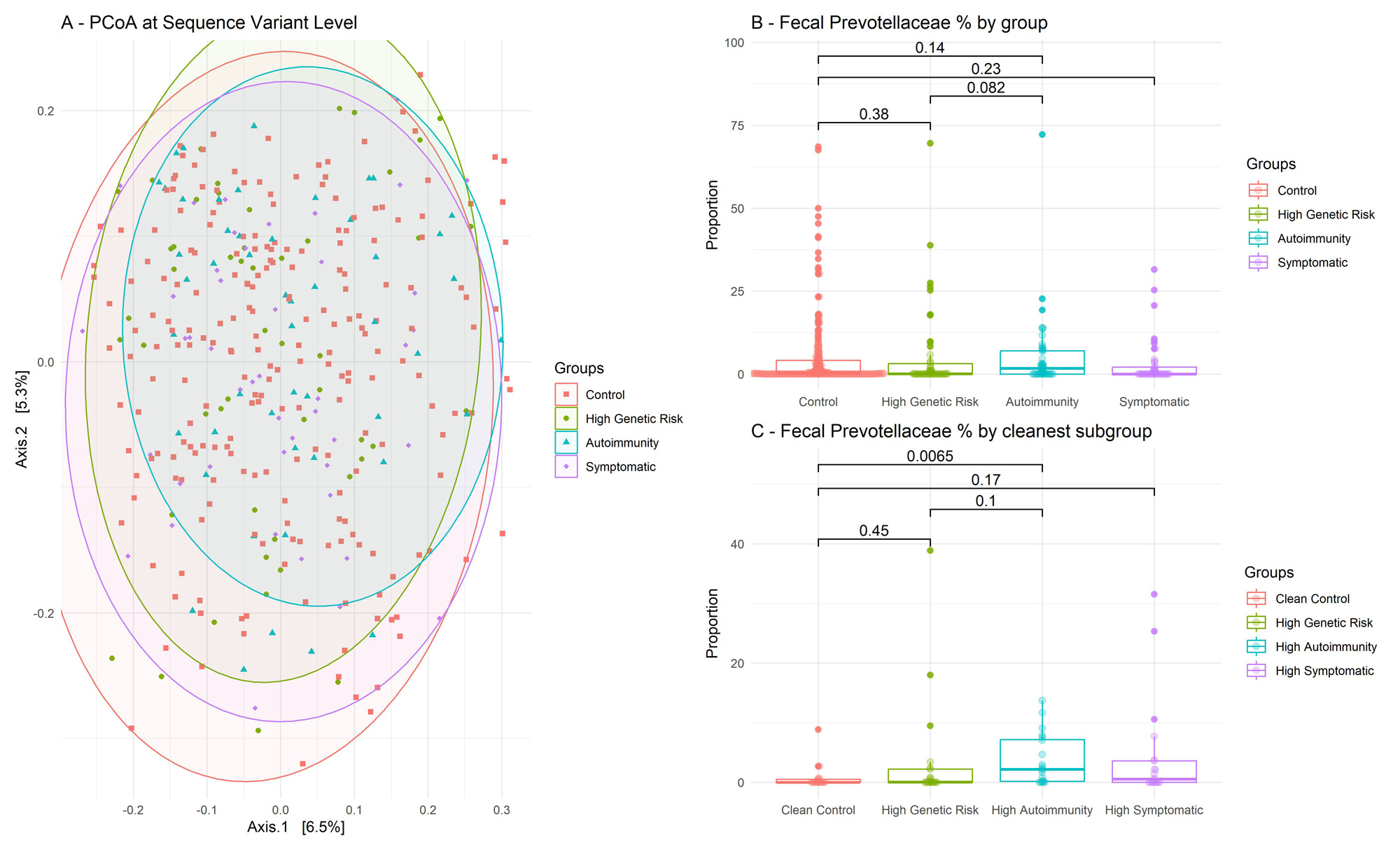
Results: A total of 372 individuals were included (Table 1). Groups had similar age, gender, and BMI. We found no group-wise clustering of 16S sequence variants (PERMANOVA, R2 = 0.0073, p = 0.81) (Figure 1A) and we found no group-differences in Prevotellaceae abundance (Figure 1B; Kruskal-Wallis p = 0.14). Results were similar after fecal cell counts correction (not shown). Aldex2 found no significant differences between groups regarding other bacterial families.
In a predefined subgroup analysis, selecting the most pronounced phenotypes in each group (i.e. highest autoantibody titers or arthralgia scores, n = 4×20) reproduced published results regarding Prevotellaceae (Figure 1C – Wilcoxon p < 0.01).
Examining biomarkers of mucosal inflammation, we found a trend for fecal calprotectin elevation in the autoimmunity stage compared to controls (p = 0.074), which was no longer apparent in the symptomatic stage.
Specific bacterial cell counts, or abundances, failed to identify intestinal ‘dysbiosis’ in pre-clinical stages of RA and are most likely not usable as clinical biomarkers under these conditions. Still, a comparison of the most pronounced phenotypes retrieved the previously reported association, which suggests a weak signal in a distinct subgroup of patients. Accounting for lifestyle characteristics, such as diet, use of antibiotics or probiotics, may reduce confounding. Potentially increased fecal calprotectin in asymptomatic RA autoimmunity could suggests an ongoing low grade mucosal inflammation of the intestine.
Conclusion: Prevotellaceae abundance, or bacterial cell counts, are associated with RA-autoimmunity only in the most pronounced phenotypes of preclinical RA stages. But in the larger overall FDR population, specific bacterial taxa were not associated with pre-clinical RA stages.
References
- Alpizar-Rodriguez, D. et al.Ann. Rheum. Dis.78,590–593(2019), www.doi.org/10.1136/annrheumdis-2018-214514
- Gilbert, B. T. P. et al.BMJ Open11,e048409(2021), www.doi.org/10.1136/bmjopen-2020-048409
SD: Standard Deviation. N: Negative. L: Low positivity (1 to 3x the norm). H: High positivity (>=3x the norm). ACPA: Anti-Citrullinated Protein Antibodies. RF: Rheumatoid Factor. Anti-ra33: anti-Ra33 autoantibodies. CSA: Clinical Suspect Arthralgia according to the EULAR definition – score ranges from 1 to 7, with cutoffs at 3 or 4. Shared Epitope: a group of MHC-II alleles conferring higher risk for RA. NA: Not Assigned.
A – PCoA : Principal Coordinate Analysis, performed at the Amplicon Sequence Variant level. On this figure, the more distant two point are, the more different they are in terms of 16S gene composition, based on Bray-Curtis index. B – Percentages of 16S sequences attributed to Prevotellaceae bacteria, per group. Wilcoxon test. C – Same panel as B, but restricted to cleaner subgroups, with high auto-antibody titers and/or high CSA scores. For readability Y-axis was cut (grey dotted line).
Measured with ELISA in fresh frozen stool. Bold points are subjects presented on panel 1C.
To cite this abstract in AMA style:
GILBERT B, TITO TADEO R, LAMACCHIA C, STUDER O, COURVOISIER D, RAES J, Finckh A. Gut Microbiome and Intestinal Inflammation in Preclinical Stages of Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/gut-microbiome-and-intestinal-inflammation-in-preclinical-stages-of-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gut-microbiome-and-intestinal-inflammation-in-preclinical-stages-of-rheumatoid-arthritis/