Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Glucagon-like peptide-1 receptor agonists (GLP-1 RA) such as semaglutide (SEM; GLP-1) and tirzepatide (TIR; GIP/GLP-1), were initially approved for type 2 diabetes management but has seen increasing use for weight loss treatment (WLT). Given the potential for weight loss to improve disease activity, function, and patient-reported outcomes (PROs) in rheumatic and musculoskeletal diseases (RMDs), understanding GLP-1 use patterns and effects is crucial.
Methods: We conducted a retrospective analysis using Q3 2024 (1/05–9/24) data from the ACR Rheumatology Informatics System for Effectiveness (RISE) registry, among RMD patients treated with SEM or TIR. Patients were categorized as new users (with ≥1 evaluation and management [E/M] visit prior to GLP-1 initiation) or prevalent users (without a prior E/M visit). Baseline included data on or before the index date. We examined baseline characteristics, RMD diagnoses, concurrent medications, and weight changes at 6, 12, and 18 months, stratified by GLP-1 type and diabetes. GLP-1 exposure was characterized as intent-to-treat (ITT). Multivariable linear regression assessed 12-month weight loss by GLP-1 type, adjusted for baseline weight, age, sex, and diabetes.
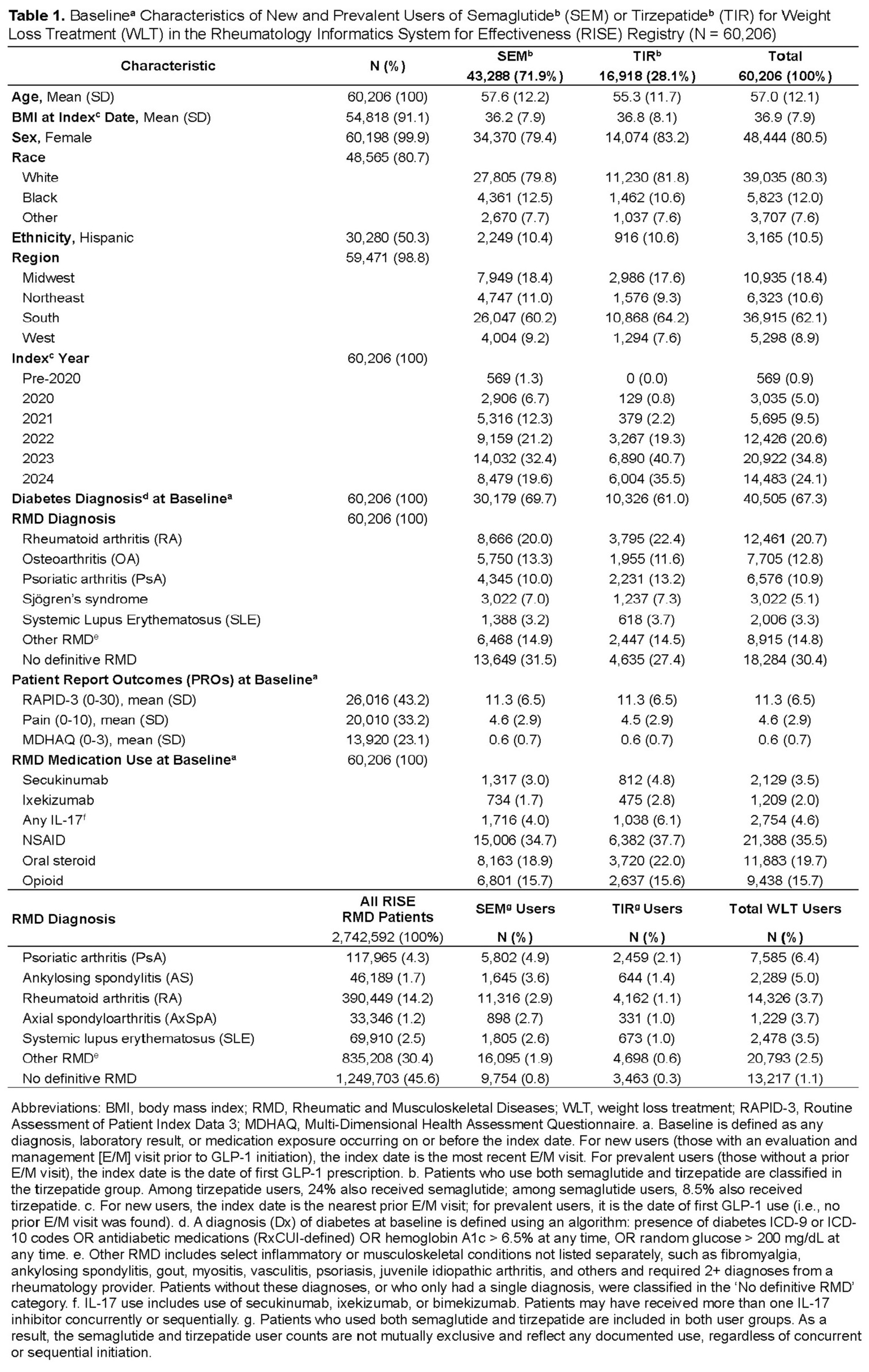
Results: Among 60,206 RMD patients treated with SEM or TIR, 67% had diabetes and 70% used SEM. Uptake was highest among patients with RA (20.7%) and OA (12.8%), with SEM more common in both. The most rapid increase occurred in 2023, accounting for 32% of all SEM and 41% of TIR use. Patients’ mean (SD) baseline BMI was 36.3 (7.9), and ~20% were on oral glucocorticoids. Across all RMD cohorts, the highest proportion of WLT use was observed in PsA patients (6.4% of all RISE PsA patients, n=117,965), followed by AS (5.0%) and RA (3.7%). Weight trajectories were evaluated among 40,006 new users of SEM or TIR. At baseline, PROs were similar across GLP-1 RA types, but diabetic users had greater disease burden, with higher RAPID-3 (mean 11.6 [SD 6.6] vs. 10.7 [SD 6.3]) and MDHAQ scores (mean 4.7 [SD 2.9] vs. 4.4 [SD 2.8]) than non-diabetic users. At 12 months, non-diabetic TIR users lost 8.0% (SD 11.3) of body weight, compared to 6.0% (SD 9.2) with SEM. Diabetic users showed a similar pattern, with TIR consistently outperforming SEM. Across GLP-1 RA types, non-diabetic users lost more weight than diabetic users. Weight loss plateaued after 12 months, with 1.0% weight regain in non-diabetic TIR users by 18 months. In models adjusting for baseline weight, age, sex, and diabetes, TIR users lost approximately 1.7% (p< 0.001) more weight at 12 months than SEM users. Patients without diabetes lost about 1.5% (p< 0.001) more weight than those with diabetes, regardless of GLP-1 RA type. No significant interactions were observed among models’ variables.
Conclusion: In this national registry of RMD patients, SEM and TIR were increasingly used and effective weight loss treatments for individuals with and without diabetes. Across both GLP-1 RAs, non-diabetic users lost more weight than diabetic users, with TIR showing greater reductions than SEM over time. Ongoing work evaluates the impact of GLP-1 therapy on disease activity, function, and PROs in RMD populations.
To cite this abstract in AMA style:
McCormick N, Zhang J, Holladay E, Xie F, Curtis J. GLP-1 Receptor Agonists to Facilitate Weight Loss and Improve Disease Activity, Pain and Function in Patients With Rheumatic and Musculoskeletal Disease: Real-World Evidence From the Rheumatology Informatics System for Effectiveness (RISE) Registry [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/glp-1-receptor-agonists-to-facilitate-weight-loss-and-improve-disease-activity-pain-and-function-in-patients-with-rheumatic-and-musculoskeletal-disease-real-world-evidence-from-the-rheumatology-info/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/glp-1-receptor-agonists-to-facilitate-weight-loss-and-improve-disease-activity-pain-and-function-in-patients-with-rheumatic-and-musculoskeletal-disease-real-world-evidence-from-the-rheumatology-info/

.jpg)
.jpg)