Session Information
Date: Sunday, November 17, 2024
Title: Abstracts: RA – Diagnosis, Manifestations, & Outcomes III: Best Day (RA Subpopulations)
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Therapeutic advances of the recent past have led to significant changes in clinical practice when treating patients with rheumatoid arthritis (RA). Since the advent of biological disease modifying anti-rheumatic drugs (DMARD), double-blind randomized controlled trials (RCT) have been the mainstay of drug development. Over time, a significant increase in placebo response (PBO) rates has been observed in these trials. We hypothesized that one element contributing to these increasing placebo responses are changing recruitment patterns with an increasing move towards geographic areas with less affluent health care systems.
Methods: We analysed the placebo arms of all available trials with reports on ACR response rates at week 12 and/or week 24. To ensure completeness of trial acquisition, we identified all PBO controlled RCTs investigating biological or targeted synthetic DMARDs in RA patients with established csDMARD background therapy until January 26th, 2024. To assess geographic distribution, we extracted the numbers of recruiting centres per country from the original articles or from clinicaltrials.gov. We then used data published by the world bank on the per capita gross national income (GNI) of each recruiting country to calculate a weighted GNI per study. We performed linear mixed model regression with a random effect on study level (to control for study heterogeneity), weighted by the number of patients per study, and adjusted for the global trend of rising GNIs over time.
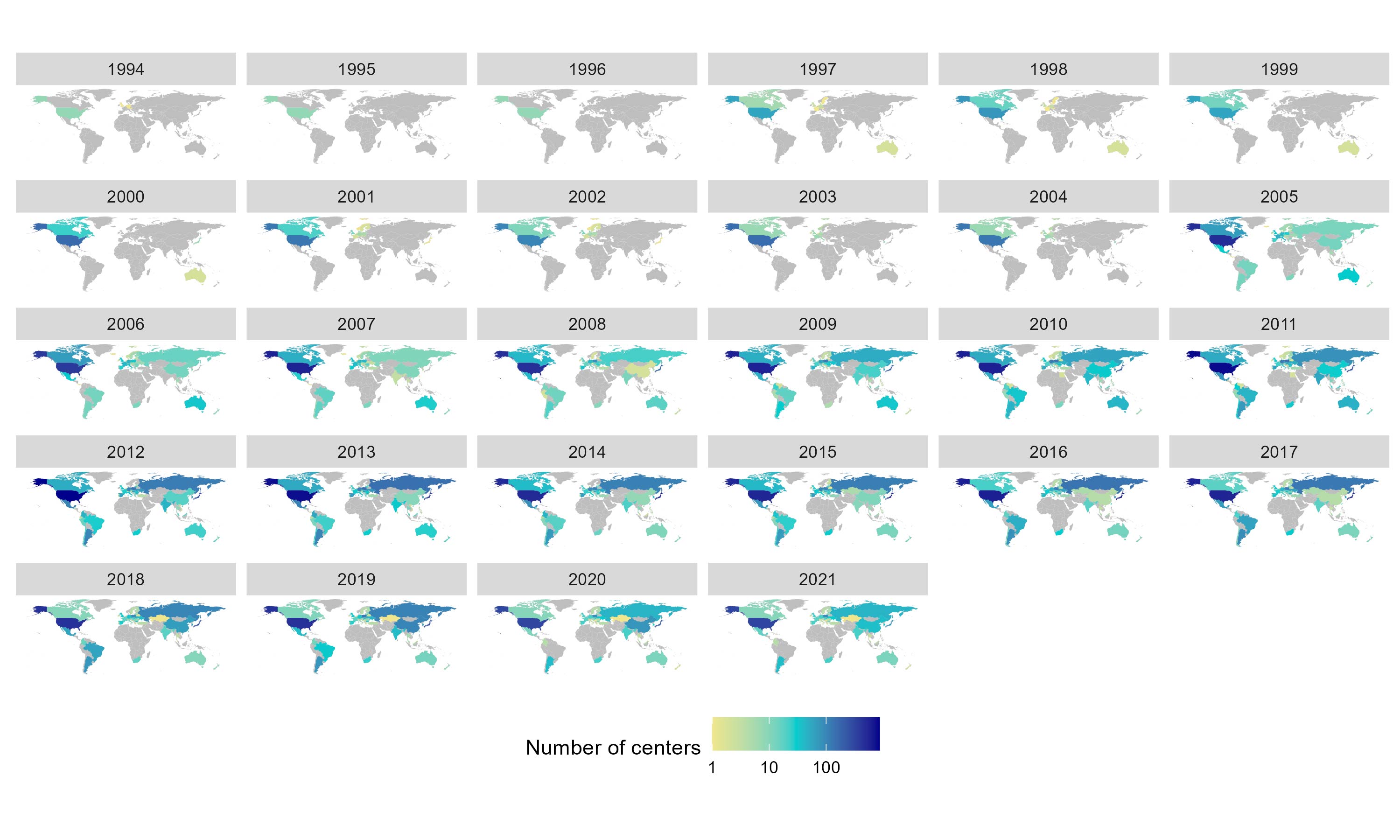
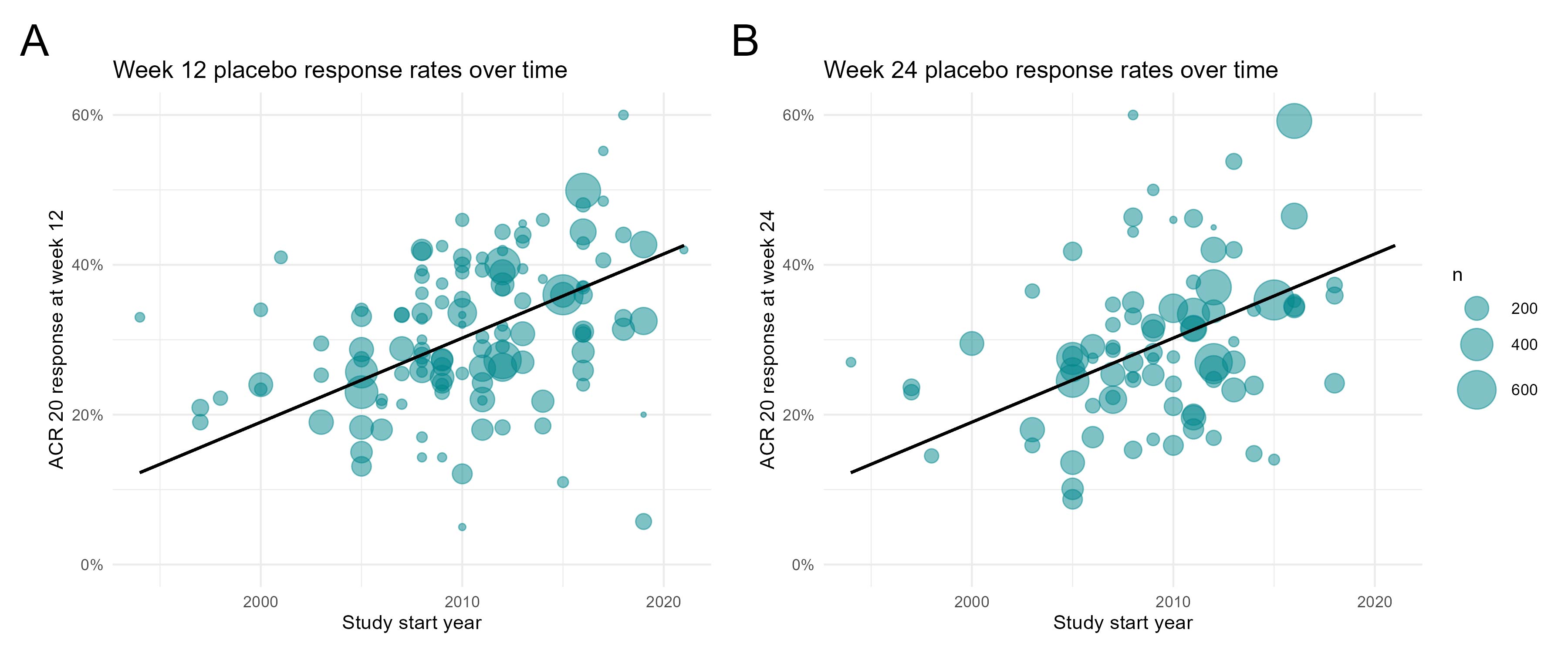
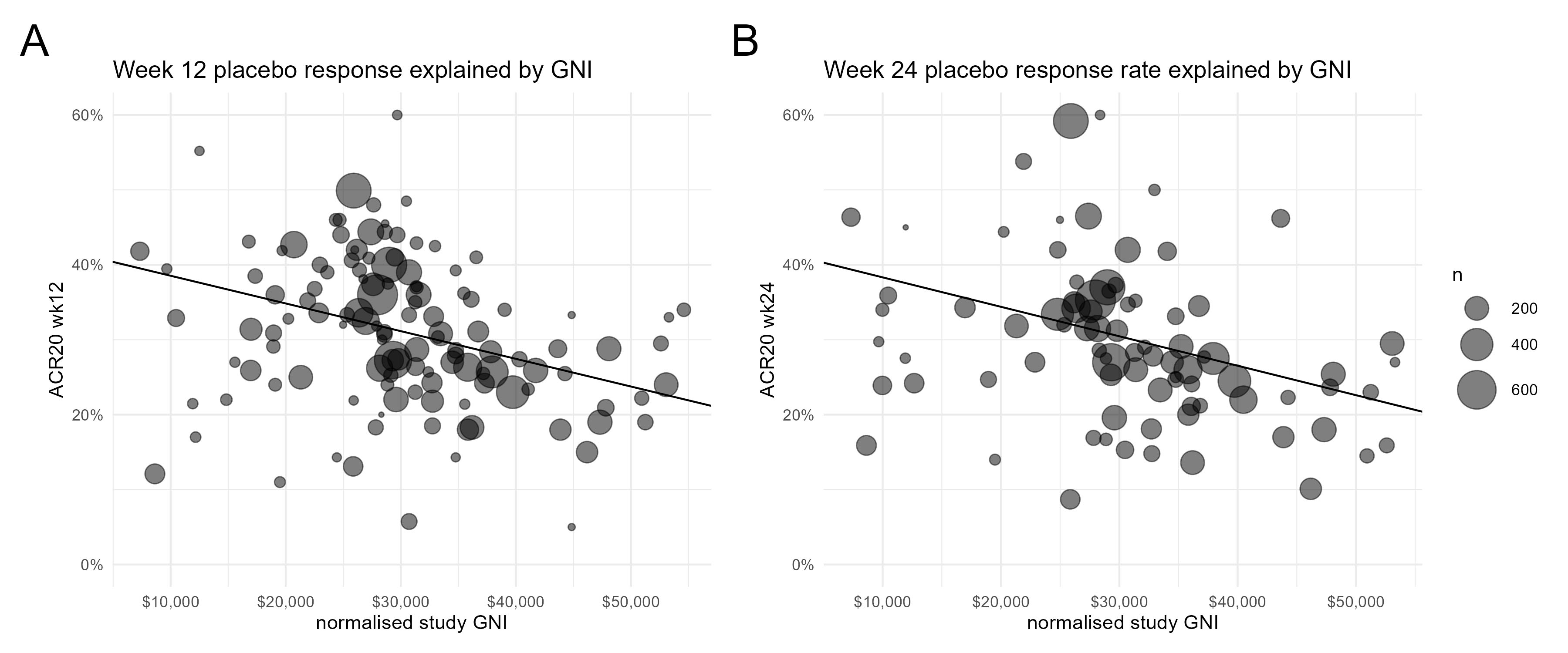
Results: For complete trial evaluation, we screened in total 13.634 articles, of which 577 were assessed in detail, with 135 articles finally fulfilling the criteria for trial analysis. Of the included studies the year of study start ranged from 1994 to 2019. Recruitment areas have expanded from initially covering only North America and Western Europe to an increasing global recruitment over time (Figure 1), with more recent studies being conducted in less affluent countries. A significant positive association of PBO ACR20% response rates with the study start year was found, at both, week 12 (n=125; 0.87±0.21;p< 0.001) and week 24 (n=83; 1.12±0.29;p< 0.001; Figure 2). We identified a strong and significant negative association of the weighted and normalised GNI per study and PBO ACR20 response rates in a study, with an estimate of -3.7% (n=125;SE:1.01;p< 0.001) per 10.000 international Dollar at week 12 (Figure 3, Panel A) and -3.93% (n=83;SE:1.20;p< 0.001) at week 24 (Figure 3, Panel B).
Conclusion: PBO rates are rising over time, in parallel to the change of geographic recruiting patterns, with more recent RCTs recruiting increasingly in less effluent countries. Our data support the hypothesis that these geographic recruitment changes are a significant contributor to the higher PBO rates observed in more recent clinical trials. One explanation may be the more limited access to health care and innovative therapies in less affluent countries, leading to preferential incentives for inclusion in clinical trials and subsequently also higher regression to the mean, which will manifest as PBO response. These effects should have implications for sponsors, investigators, patients, health care providers and political stakeholders.
To cite this abstract in AMA style:
Kerschbaumer A, Steiner M, Pruckner P, Smolen J, Aletaha D. Global Recruiting Patterns Are Associated with Placebo Response Rates in Clinical Trials of Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/global-recruiting-patterns-are-associated-with-placebo-response-rates-in-clinical-trials-of-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/global-recruiting-patterns-are-associated-with-placebo-response-rates-in-clinical-trials-of-rheumatoid-arthritis/