Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The most common types of ANCA-associated vasculitis, granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA), can affect a range of organs, including the kidneys, respiratory system, skin, nervous system, and eyes.1 The phase 3 ADVOCATE trial (NCT02994927) compared avacopan vs. a prednisone taper to treat patients with GPA or MPA.2 Patients receiving avacopan had improvements in sustained remission with less glucocorticoid (GC) exposure and GC-related toxicity than those receiving a prednisone taper.2 Data regarding kidney, lung, and ear, nose, and throat involvement from ADVOCATE were reported previously,2,3 prompting the need for an analysis of the general, skin, nerve, and eye outcomes in patients with GPA or MPA.
Methods: This post hoc analysis of the ADVOCATE trial reports rates of active general, nervous system, mucous membranes/eyes, and skin involvement based on the Birmingham Vasculitis Activity Score at weeks 4, 26, and 52, and changes from baseline to week 52.
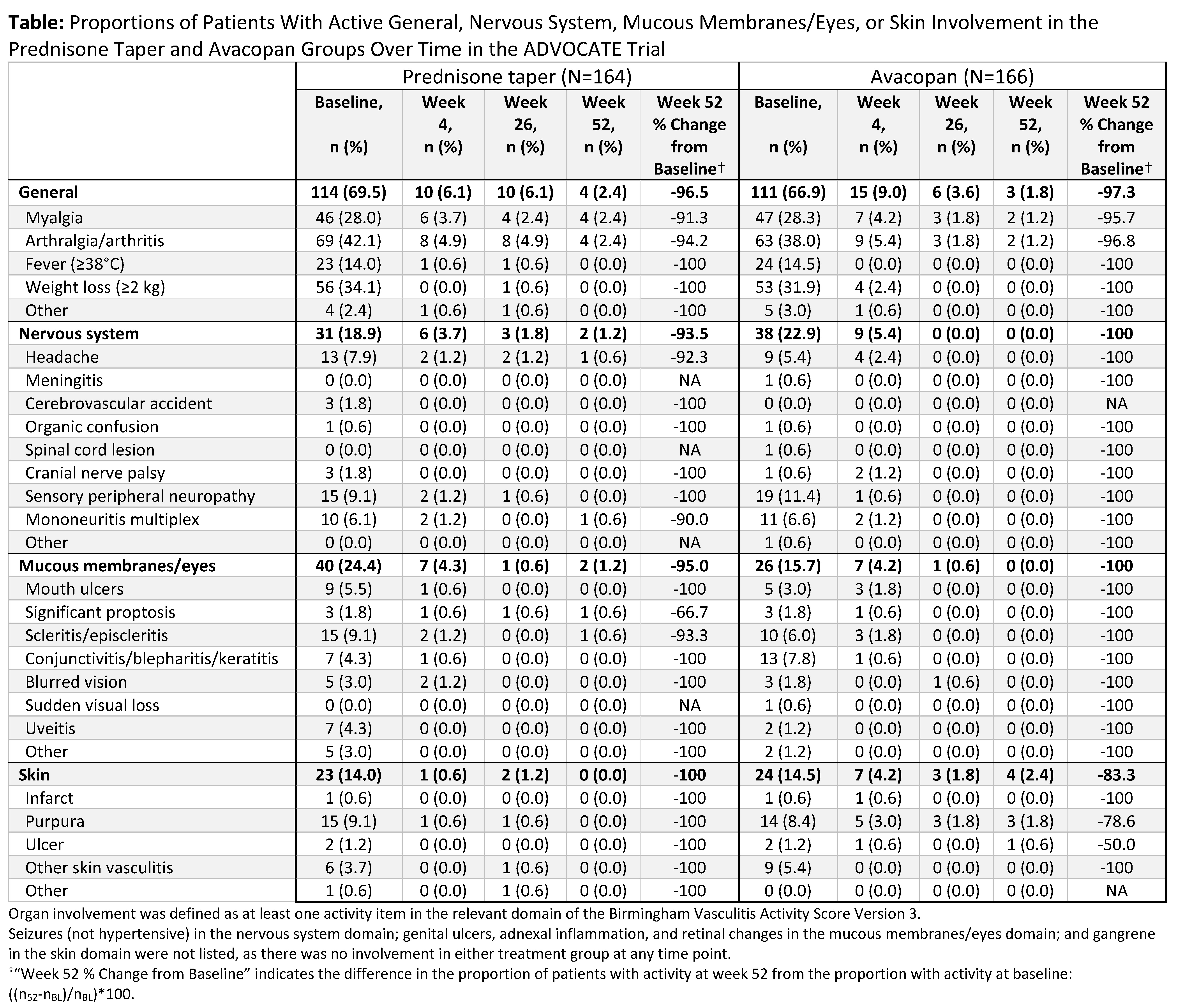
Results: In the 330-patient ADVOCATE trial, active involvement of the general, nervous system, mucous membranes/eyes, and skin domains affected 68.2% (n=225), 20.9% (n=69), 20.0% (n=66), and 14.2% (n=47) of patients, respectively; most patients had at least one of these manifestations (Table). Similar and substantial improvements in the control of active disease in these domains were achieved in both groups. Reductions from baseline to week 52 in the proportion of patients with active manifestations in avacopan vs. prednisone taper groups, respectively, were 97.3% vs. 96.5% (general), 100% vs. 93.5% (nervous system), 100% vs. 95% (mucous membranes/eyes), and 83.3% vs. 100% (skin).
Conclusion: In the ADVOCATE trial, treatment with either avacopan or a prednisone taper was associated with the reversal of nearly all active general, nervous system, mucous membranes/eyes, and skin manifestations of GPA or MPA.
References:
1. Koldingsnes W, Nossent H. Rheumatology (Oxford) 2002;41(5):572-581.
2. Jayne DRW, et al. N Engl J Med 2021;384(7):599-609.
3. Specks U, et al. Am J Resp Crit Care Med 2022;205:A4780.
To cite this abstract in AMA style:
Hajj-Ali R, Geetha D, Luqmani R, Pagnoux C, Trimpe D, Jayne D, Merkel P. General, Nervous System, Eye, and Skin Involvement in the Phase 3 Trial of Avacopan for the Treatment of ANCA-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/general-nervous-system-eye-and-skin-involvement-in-the-phase-3-trial-of-avacopan-for-the-treatment-of-anca-associated-vasculitis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/general-nervous-system-eye-and-skin-involvement-in-the-phase-3-trial-of-avacopan-for-the-treatment-of-anca-associated-vasculitis/