Session Information
Date: Tuesday, November 9, 2021
Title: Systemic Sclerosis & Related Disorders – Clinical Poster III (1836–1861)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Evaluation of skin is central in clinical management of systemic sclerosis (SSc). Due to the COVID-19 pandemic remote consultations were widely implemented, which inevitably limited skin assessment using the modified Rodnan Skin Score (mRSS). In order to monitor skin during the pandemic, we developed and validated the PASTUL (Patient self-Assessment of Skin Thickness in Upper Limb) questionnaire. (Spierings J et al Ann Rheum Dis. 2021: doi: 10.1136/annrheumdis-2020-219775. PMID: 33514506) We now explored utility of the PASTUL questionnaire in trials to translate the benefit of its use in clinical practice back into research in different subgroups of patients.
Methods: Patients included in the validation study were divided into subgroups: disease duration < 4 and > 4 years, males and females, diffuse cutaneous SSc (dcSSc) and limited cutaneous SSc (lcSSc), antibody profiles and age categories. The PASTUL questionnaire specifies a grading of skin (normal (0), mild (1), moderate (2), severely (3) thickening) at 8 sites corresponding to mRSS. To simplify the assessment, maximum score of an anatomical area was asked. Correlation between PASTUL, mRSS, Scleroderma Skin Patient reported Outcome (SSPRO) and Scleroderma Health Assessment (SHAQ) was assessed using Pearson’s correlation coefficient (0-0.19 = negligible, 0.2-0.39 = weak, 0.4-0.59 = moderate, 0.6-0.79 = strong, 0.8-1.0 = very strong).
Results: Of the 104 patients included, 86% was female, 55 (53%) had lcSSc and 49 (47%) dcSSc, 75 (72%) had a disease duration > 4 years, 70 (67%) were older than 50 years. Mean age was 57 years (SD 12), mean disease duration 11 years (SD 9), mean SHAQ 1.4 (SD 0.8) and mean SSPRO 48 (SD 27). MRSS was measured in 78 patients (75%), mean mRSS was 14 (SD 10). Table 1 shows characteristics of the different groups.
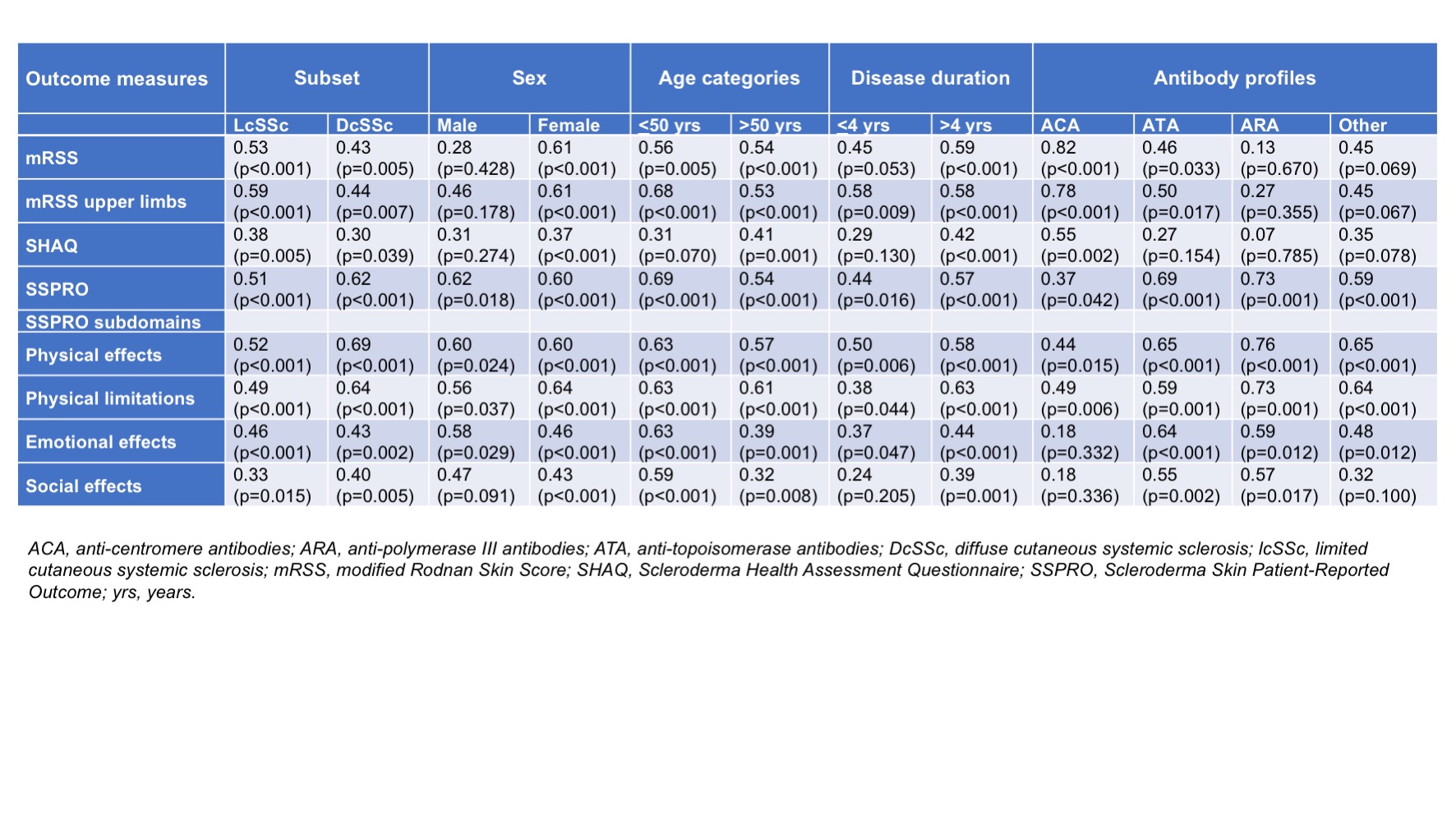
An overview of correlations between PASTUL and mRSS, SSPRO and SHAQ scores in the subgroups is provided in Table 2. Correlation between PASTUL and mRSS was very strong in ACA positive patients (r=0.82) and strong in females (r=0.61). Correlations between PASTUL and mRSS were stronger in lcSSc compared to dcSSc (r=0.53 vs 0.43), in females compared to males (r=0.61 vs 0.28), in patients with disease duration > 4 years (r=0.45 vs 0.59) and ACA positives compared to patients with ATA, ARA or other antibodies (r=0.82, 0.46, 0.13 and 0.45, respectively). PASTUL was moderately correlated with SHAQ in ACA positives (r=0.55) only. SSPRO and subdomains correlated weakly to moderately in this group, in contrast to other subgroups in which a stronger correlation between SSPRO and PASTUL was found.
Conclusion: Almost all subgroups showed moderate to strong correlations between PASTUL and mRSS and SSPRO. Correlations between PASTUL scores and mRSS were strongest in ACA positives and females, while ARA positive patients showed strongest PASTUL correlation with SSPRO. These correlations support potential utility of PASTUL as outcome measure and further validation in larger cohorts will define the role of PASTUL in future clinical trials.
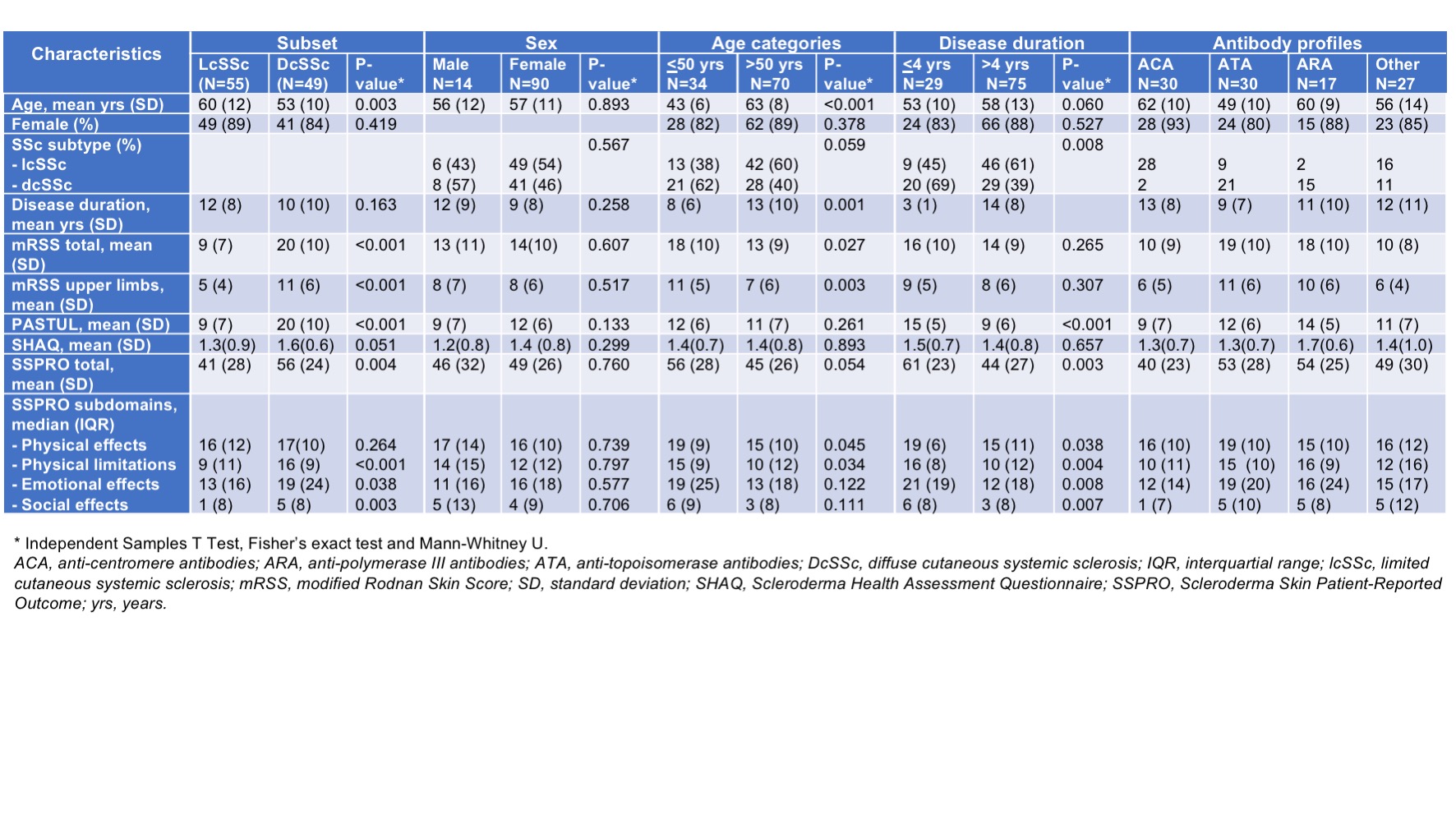 Table1.jpeg”Table 2. Correlations (Pearson’s correlation coefficient) of PASTUL with other outcome measures in different subgroups
Table1.jpeg”Table 2. Correlations (Pearson’s correlation coefficient) of PASTUL with other outcome measures in different subgroups
To cite this abstract in AMA style:
Spierings J, Ong V, Denton C. Further Construct Validation of the [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/further-construct-validation-of-the/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/further-construct-validation-of-the/