Session Information
Date: Wednesday, November 15, 2023
Title: Abstracts: Systemic Sclerosis & Related Disorders III: Clinical Trials
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Systemic Sclerosis (SSc) is an autoimmune disease characterized by vascular damage, inflammation, and fibrosis of the skin and organs, with no approved disease modifying treatments. The available treatment options are limited to systemic sclerosis-associated interstitial lung disease to slow the decline in lung function. Accordingly, there remains a serious unmet medical need for safe and effective therapeutics for the SSc population. FT011, a G protein-coupled receptor 68 (GPR68) proton sensing antagonist is being developed as a novel inhibitor of pro-fibrotic and inflammatory pathways.
Methods: This was a Phase II, multi-center, randomized, double blind, placebo-controlled study of the pharmacokinetics, pharmacodynamic effects, and safety, of oral FT011 in patients with diffuse SSc (dSSc). The study randomized 30 participants (n = 10/group) to placebo, FT011 200 mg or FT011 400 mg taken once daily (OD) for 12 weeks. ClinicalTrials.gov Identifier: NCT04647890
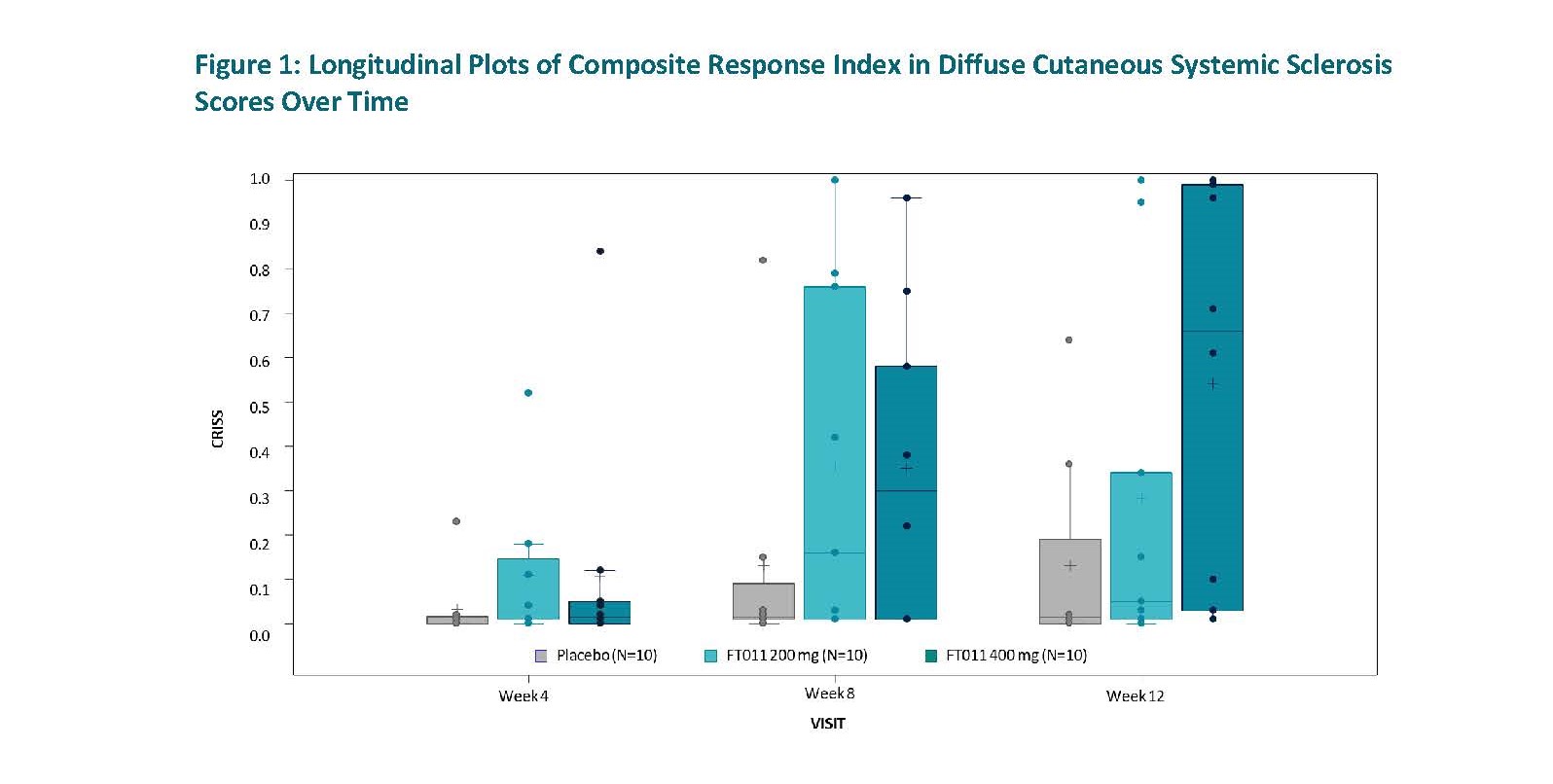
Results: Patients were a mean age of 51 years, white and the majority (73%) female. Treatment with FT011 400 mg OD for 12 weeks resulted in significant and clinically meaningful improvements in the American College of Rheumatology Combined Response Index in Diffuse Cutaneous Systemic Sclerosis (ACR-CRISS) score and its composites.
By week 12, 60% of patients in the FT011 400 mg arm were classified as clinical responders with improvement in ACR-CRISS (defined by predicted probability ≥0.60)1 vs 10.0% in the placebo arm (nominal p-value = 0.046). Median ACR-CRISS score was 0.660 vs 0.015, FT011 400 mg vs placebo, respectively. The mean ACR-CRISS score was 0.542 in the FT011 400 mg arm vs 0.131 in the placebo arm (nominal p-value = 0.019) (Figure 1).
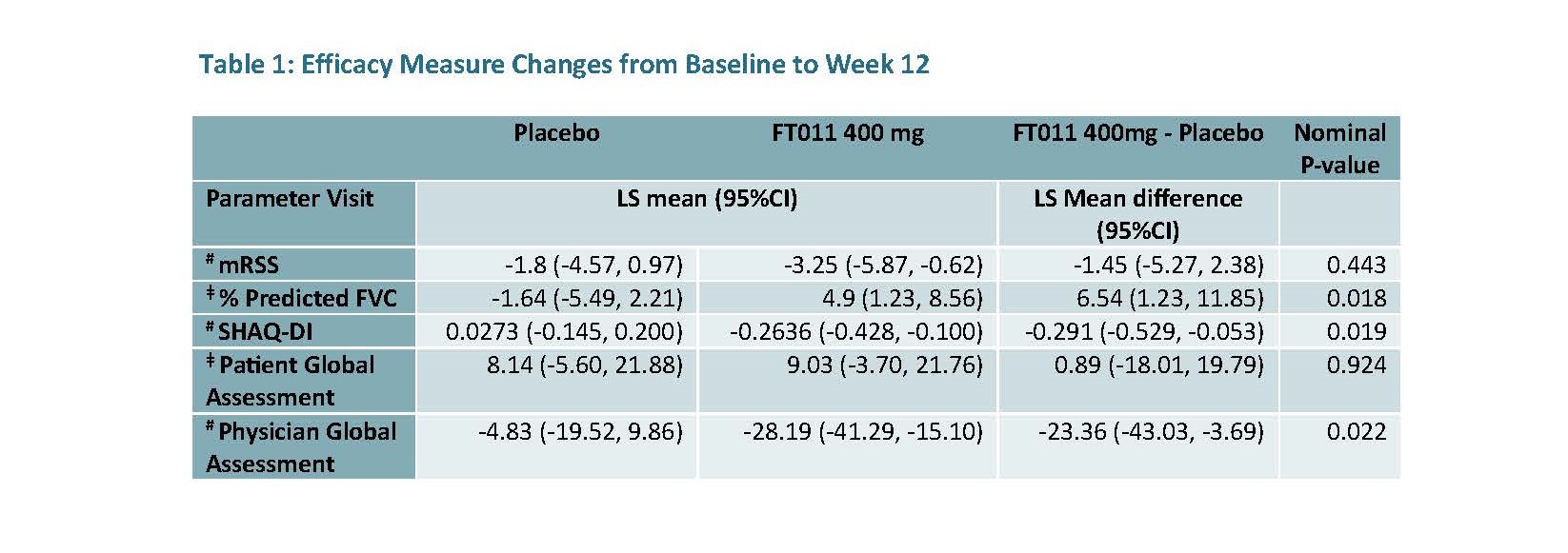
FT011 also led to significant and clinically meaningful improvements across multiple efficacy measures including % predicted Forced Vital Capacity (FVC), Scleroderma Health Assessment Questionnaire-Disability Index (SHAQ-DI) and physician global assessments (Table 1).
50% of patients in the FT011 400 mg arm exceeded the % predicted FVC minimally important clinically difference (MICD) (defined as a change of >3.3 – 5.3%)2 vs no patients in the placebo arm meeting these criteria. Furthermore, 60% of patients in the FT011 400 mg arm met the MICD for SHAQ-DI (defined as a change of −0.13)3 vs 22% in the placebo arm.
Compared to placebo, FT011 demonstrated numerically larger improvements from baseline to week 12 for mRSS and patient global assessment, however this was not significant.
FT011 was safe and well tolerated, with no differences in drug-related treatment-emergent adverse events between placebo and active treatment groups. There were no serious adverse events reported, nor any adverse events resulting in study drug interruption, withdrawal, or discontinuation.
Conclusion: These results demonstrate promising efficacy and safety data for FT011 OD after 12 weeks of treatment and therefore warrants a confirmatory phase III study to assess its potential to improve this debilitating condition.
References
1 Khanna D et al. Arthritis Rheumatol. 2016 Feb;68(2):299-311.
2 Kafaja S, et al. Am J Respir Crit Care Med. 2018 Mar 1;197(5):644-652.
3 Daste C, et al. Semin Arthritis Rheum. 2019 Feb;48(4):694-700.
CRISS: Composite Response Index in Diffuse Cutaneous Systemic Sclerosis. IQR: Interquartile Range. The CRISS score is ranged from 0.0 to 1.0 where higher scores indicate greater improvement.
Symbol inside the box: Mean. Horizontal line inside the box: Median. Bottom and top of box: 25th and 75th percentiles, respectively. Bottom and top whiskers: Extreme value within 1.5 IQR. Circle: individual participant’s value.
ǂ Positive value indicates improvement and # Negative value indicates improvement.
A formal sample size calculation for efficacy endpoints was not conducted for this study; the results are considered exploratory.
To cite this abstract in AMA style:
Denton C, Stevens W, Kruger N, Papadimitriou M, Khong F, Bradney M, Kelly D, Lafyatis R. FT011 for the Treatment of Systemic Sclerosis. Results from a Phase II Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/ft011-for-the-treatment-of-systemic-sclerosis-results-from-a-phase-ii-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ft011-for-the-treatment-of-systemic-sclerosis-results-from-a-phase-ii-study/