Session Information
Date: Sunday, November 8, 2020
Title: Pediatric Rheumatology – Clinical Poster II: Systemic JIA, Autoinflammatory, & Scleroderma
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Monogenic autoinflammatory diseases (AID) are caused by innate immune dysregulation resulting in systemic inflammation and variable organ-specific clinical manifestations. The Translational Autoinflammatory Disease Section (TADS) studies patients with rare and early-onset AIDs, such as the IL-1 mediated diseases neonatal onset multisystem inflammatory disease (NOMID) and deficiency of IL-1 receptor antagonist (DIRA), the interferon (IFN) mediated diseases chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) and STING associated vasculopathy with onset in infancy (SAVI), and patient with yet undifferentiated AIDs. We aim to identify the frequency of genetically defined diagnoses, demographic information and mortality rates in patients enrolled in TADS Natural History Protocol.
Methods: All patients were consented to an IRB-approved natural history protocol (NCT02974595) and underwent a standard procedure of clinical and laboratory evaluation. For genetic investigation, most patients had Sanger sequencing, whole exome (WES) or genome sequencing (WGS) performed. Patients with IFN-mediated diseases had whole blood interferon scores performed to assess disease-associated flare status. Patient information was compiled into a clinical database that includes variables such as demographic features, diagnosis, clinical manifestations, disease outcome and gene affected.
Results: Since 2003, 471 patients have been enrolled in the AID natural history protocol. Genetic diagnoses were assigned to 197/471 (41.8%). The cohort consists of 194 males (41.1%), 277 females (58.8%). Demographics and outcomes of genetic diagnoses with more than 3 confirmed patients are depicted in Table 1. These included patients with CANDLE (n=17), SAVI (n=24), DIRA (n=9), NOMID (n=60), IL-18 pulmonary alveolar proteinosis and macrophage activation syndrome (IL-18PAP-MAS, n=8), Aicardi-Goutières syndrome (AGS, n=7), NEMO-deleted exon 5 autoinflammatory syndrome (NEMO-NDAS, n=10), and SAMD9L-associated autoinflammatory disease (SAMD9L-SAAD, n=6). Among patients with undifferentiated AID and genetic diagnoses with less than 3 confirmed cases (Other genetic diagnosis)(Table 1), we have identified two patients with LYN-kinase associated AID, two with dysferlin associated AID, one patient with Majeed syndrome and another with CARD14-mediated psoriasis (CAMPS). We have also identified 5 patients with monogenic forms of MAS, including 3 patients with NLRC4-MAS and 2 with CDC42-AID. Interestingly, 7 patients had 2 genetic diagnoses, including one patient with NEMO-NDAS and LRBA-deficiency. Diseases showing the highest mortality in our cohort were SAMD9L-SAAD (33.3%), SAVI (21%), IL-18PAP-MAS (20%), and CANDLE (11.8%).
Conclusion: A comprehensive characterization of an AID cohort revealed a variety of monogenic AIDs in 42% of the patients. Future studies to assess response to treatment, end organ damage and disease progression are needed.
Funding for this study was provided in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
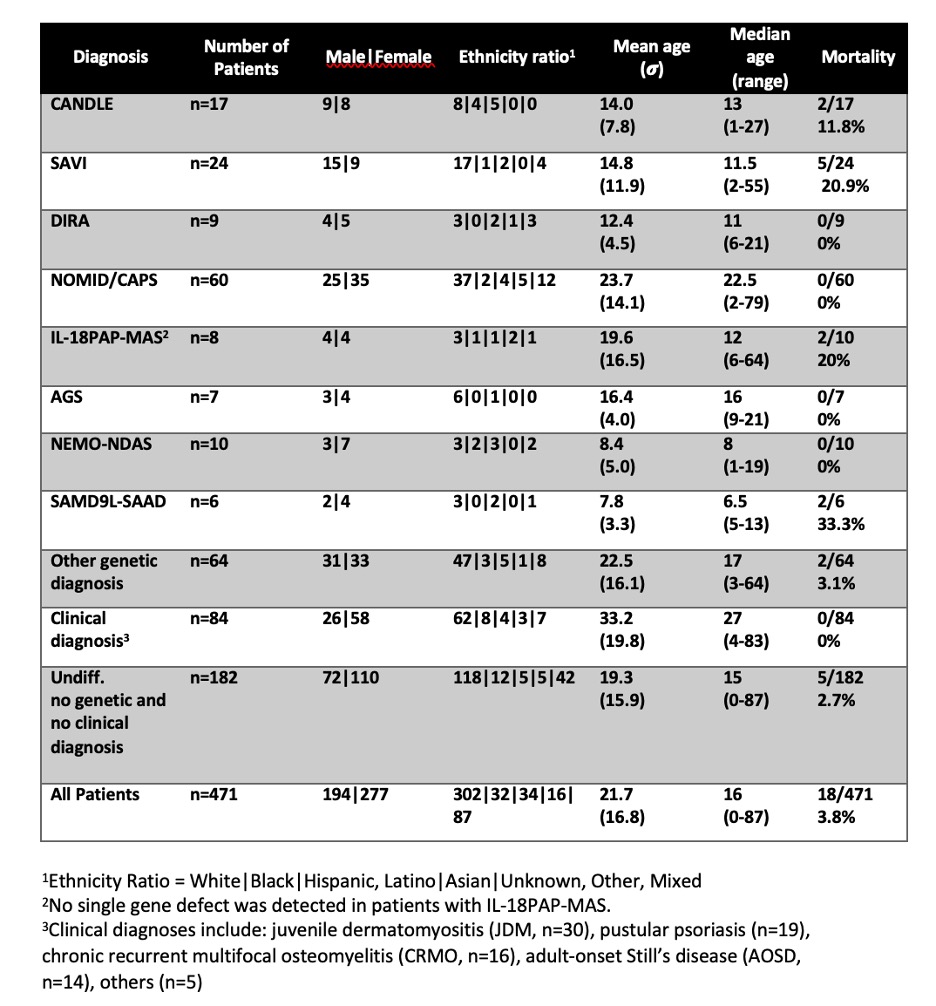 Table 1. Demographic Features of Patients Enrolled in an Autoinflammatory Disease Natural History Protocol
Table 1. Demographic Features of Patients Enrolled in an Autoinflammatory Disease Natural History Protocol
To cite this abstract in AMA style:
Honer K, Johnson K, Souto Adeva G, Montealegre Sanchez G, Wade J, Mitchell J, Townsend K, de Jesus A, Goldbach-Mansky R. Frequency of Genetic Diagnosis in an Autoinflammatory Disease Natural History Protocol Cohort of Patients [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/frequency-of-genetic-diagnosis-in-an-autoinflammatory-disease-natural-history-protocol-cohort-of-patients/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/frequency-of-genetic-diagnosis-in-an-autoinflammatory-disease-natural-history-protocol-cohort-of-patients/
