Session Information
Date: Monday, November 18, 2024
Title: T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
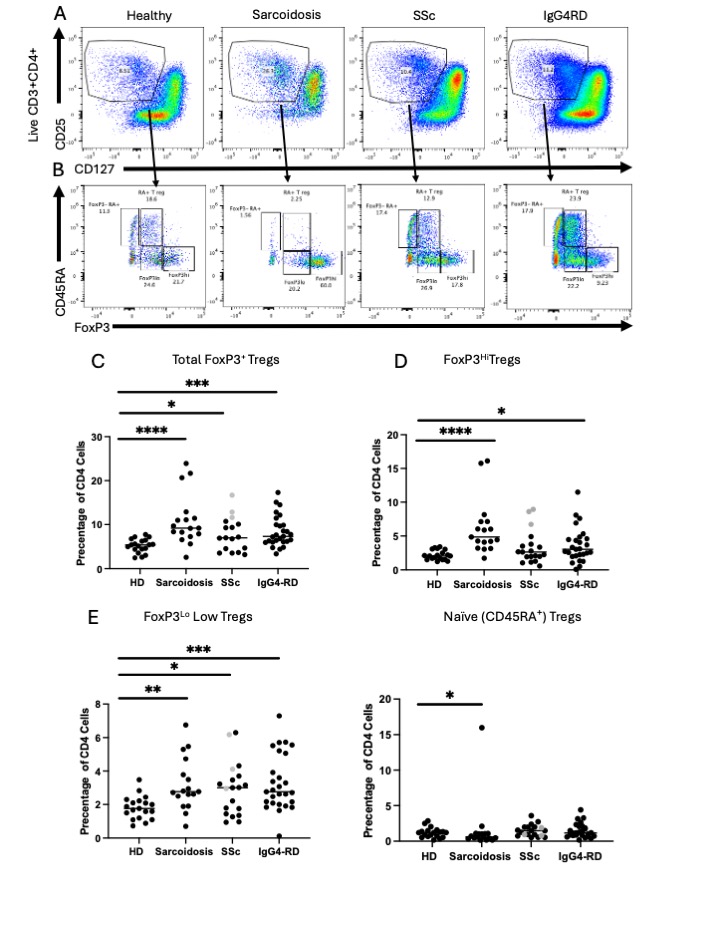
Background/Purpose: The absence of regulatory T cells (Tregs) results in multiorgan autoimmunity in the context of monogenic “Tregopathies”, but their role in mediating polygenic autoimmune diseases remains less clearly defined. Previous studies in systemic sclerosis (SSc), IgG4-related disease (IgG4-RD), and sarcoidosis, three presumptive autoimmune diseases characterized by chronic inflammation and fibrosis, have shown conflicting results regarding Treg quantities and functionality. Precise quantification of human Tregs is challenged by heterogeneity (which remains incompletely resolved) and the gradient of expression of multiple lineage characterizing or defining markers such as CD25, CD127, and FoxP3. The most common approach used to subset human Tregs relies on CD45RA, CD25, and FoxP3. However, the omission of CD127 from most studies using this approach limits our understanding of how “FoxP3Lo” cells really differ from “FoxP3Hi” cells and what their respective relationships to autoimmunity are. In the present study, we investigated the FoxP3+ T cell composition and phenotypes across SSc, IgG4-RD, and sarcoidosis using spectral flow cytometry and RNA sequencing.
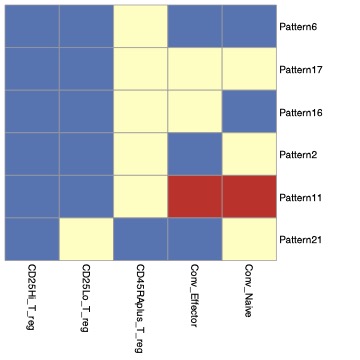
Methods: We used intracellular spectral flow cytometry to examine Treg numbers and subsets in PBMC’s from patients with SSc (n=17, median age 55; 9 diffuse, 7 limited, 1 sine), IgG4RD (n=27, median age 65, all active, untreated at the time of phlebotomy but requiring immunosuppression), sarcoidosis (n=17, median age 66, all with stable disease not requiring immunosuppression) and healthy donors (HD, n=19, median age 64). We used bulk RNA sequencing of FACS purified T cell populations to better understand the transcriptional differences between subsets.
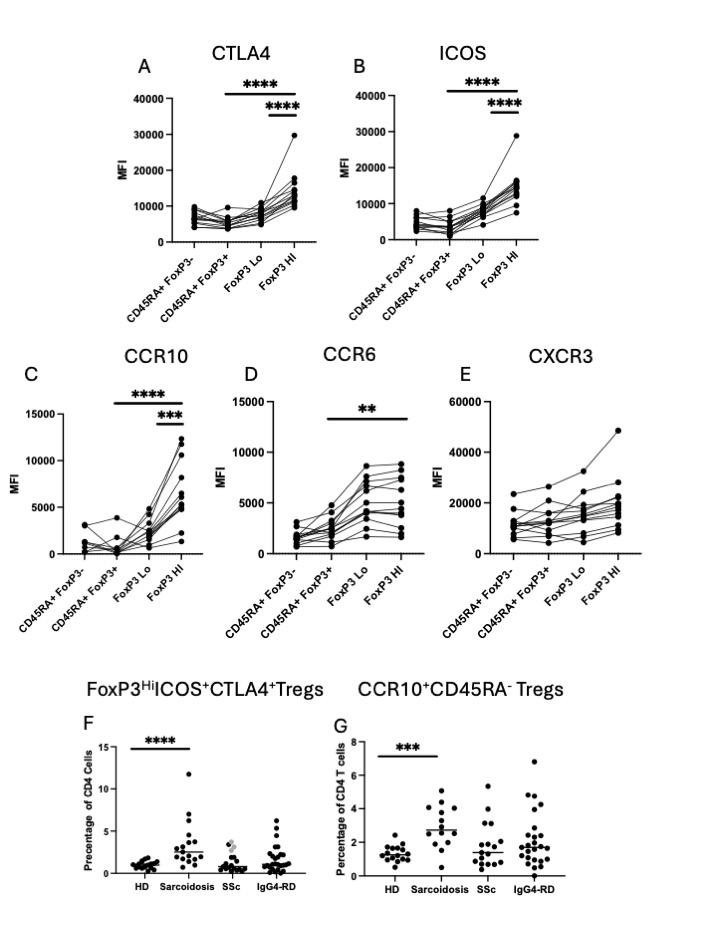
Results: Total FoxP3+ T cells and FoxP3Lo T cells were expanded in SSc, IgG4-RD, and sarcoidosis whereas FoxP3Hi T cells are more specifically expanded in sarcoidosis and only a fraction of IgG4-RD patients. ICOS and CTLA4 are expressed at highest levels in the FoxP3Hi subset and ICOS+ CTLA4+ FoxP3Hi Tregs were uniquely expanded in patients with sarcoidosis. The chemokine receptor CCR10 is nearly exclusively expressed in the FoxP3Hi Treg subset, in contrast to CXCR3 and CCR6. CCR10+ Tregs are expanded only in patients with sarcoidosis. Despite these protein level differences, the transcriptional differences between CD127LoCD25Hi and CD127LoCD25Lo subsets are starkly limited. There is a trend towards an inverse relationship between FoxP3Hi Treg numbers and disease severity in IgG4-RD.
Conclusion: We show that T cells expressing high levels of CD25, FoxP3, ICOS, and CTLA4, important mediators of Treg suppression, are uniquely expanded in patients with sarcoidosis but not in SSc and IgG4-RD. In contrast, CD127Lo CD25Lo FoxP3Lo T cells are expanded more ubiquitously across these autoimmune cohorts. FoxP3Hi Treg expansion in our cohorts correlates with mostly self-limited disease in the context of sarcoidosis or less severe disease in the setting of IgG4-RD. Further studies are needed to understand how different clinical factors across cohorts contribute to these differences in Treg expansion.
To cite this abstract in AMA style:
Yockey L, Guy T, Gadiraju C, Doyle I, Akaa J, Puri A, Wallace Z, Katz G, Montesi S, Stone J, Castelino F, Pillai S, Luster A, Mahajan V, Perugino C. FoxP3Hi CTLA4+ ICOS+ Regulatory T Cells Are Expanded in Patients with Sarcoidosis but Not Systemic Sclerosis or IgG4-Related Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/foxp3hi-ctla4-icos-regulatory-t-cells-are-expanded-in-patients-with-sarcoidosis-but-not-systemic-sclerosis-or-igg4-related-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/foxp3hi-ctla4-icos-regulatory-t-cells-are-expanded-in-patients-with-sarcoidosis-but-not-systemic-sclerosis-or-igg4-related-disease/