Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Kawasaki disease (KD) is an acute, febrile illness of childhood, associated with cardiac inflammation and vasculitis of coronary arteries and sometimes other medium sized vessel. Follistatin-like protein 1 (FSTL-1) is markedly elevated in the serum of human patients with aneurysms in KD and appears to play a major role in cardiac physiology, however, the role of FSTL-1 in cardiac inflammation is unclear. FSTL-1 protein has been found to have an anti-inflammatory role in murine models of inflammatory arthritis. We sought to determine whether FSTL-1 deficiency would alter inflammatory responses in a mouse model of Kawasaki disease, as well as how T cell signaling was altered in the presence of FSTL-1 protein in culture.
Methods: Knockout (KO) of the FSTL-1 gene was achieved by using tamoxifen regulated Cre/loxP system, and verified by ELISA and RT-PCR. KD was induced via the lactobacillus casei cell wall extract (LCWE) murine model of KD. Post LCWE cytokine production was measured by multiplex immunoassay, and FSTL-1 levels by ELISA and Western blot. Inflammatory response in acute KD was quantified by blinded pathology review of cardiac histology and immunohistochemistry for CD3 and CD11c. Bulk RNA sequencing of cardiac tissue in knock out vs wild type mice was performed as well. Measurement of phosphorylation of multiple T cell receptor downstream proteins was performed via flow cytometry using stimulated T cells and other peripheral mononuclear cells.
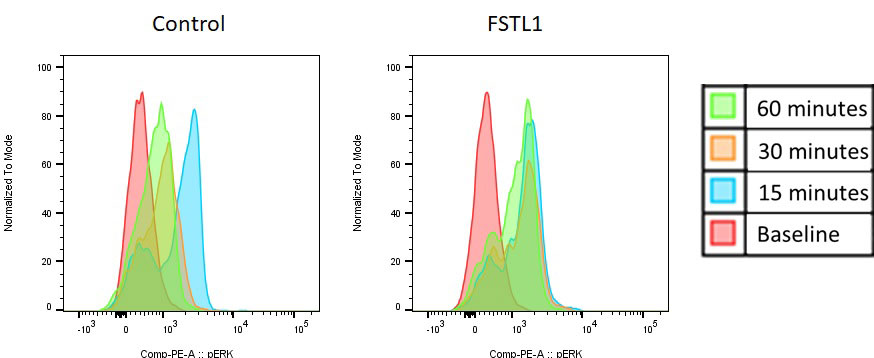
Results: FSTL-1 serum levels were elevated four fold immediately following induction of KD and correlated with histologic disease severity and CD3 tissue infiltration. FSTL-1 gene expression was upregulated in cardiac tissue at the acute phase of inflammation. Measurement of glycosylated and non-glycosylated FSTL-1 protein in the cardiac tissue and serum showed upregulation of non-glycosylated FSTL-1 in the heart, while non-glycosylated FSTL-1 was not released systemically. Serum cytokine response was unchanged in FSTL-1 KO mice vs. WT mice in the immediate acute phase of disease. FSTL-1 deficiency was associated with worsened histologic inflammation and CD3 and CD11c tissue infiltration (p< 0.01). Bulk RNA sequencing revealed several highly downregulated genes with FSTL-1 knockout associated immunologic function and also with receptor recycling functions. Analysis of phosphorylation of multiple proteins downstream of the T cell receptor suggested alterations in the kinetics (ie, duration of activation) of ERK phosphorylation.
Conclusion: Knockout of FSTL-1 in the mouse model of Kawasaki disease resulted in increased inflammatory infiltrate in the peri-aortic and coronary tissue, and FSTL-1 expression in wild type mice appeared correlated with degree inflammation. This suggests a regulatory role for FSTL-1 in terms of inflammation in the heart. Altered gene expression of transcripts related to immune function and receptor recycling (endocytosis), along with altered kinetics of T cell receptor signaling may suggest that FSTL-1 may alter how surface receptors transmit down-stream signal, specifically in terms of sustained vs intermittent signal transmission.
To cite this abstract in AMA style:
Amezcua M, Huang J, Chen M, Escalona R, Dick E, Gorelik M. Follistatin-like Protein 1 Alters T Cell Receptor Signaling Dynamics in Vitro, While Expression in Vivo Correlates with Disease and Deficiency Increases Acute Inflammation in a Mouse Model of Kawasaki Disease [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/follistatin-like-protein-1-alters-t-cell-receptor-signaling-dynamics-in-vitro-while-expression-in-vivo-correlates-with-disease-and-deficiency-increases-acute-inflammation-in-a-mouse-model-of-kawasaki/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/follistatin-like-protein-1-alters-t-cell-receptor-signaling-dynamics-in-vitro-while-expression-in-vivo-correlates-with-disease-and-deficiency-increases-acute-inflammation-in-a-mouse-model-of-kawasaki/