Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Idiopathic inflammatory myopathies (IIM) – dermatomyositis (DM), polymyositis, and inclusion body myositis – are poorly understood autoimmune muscle disorders. Isolation of relevant immune cell populations is made difficult by most protocols requiring fresh tissue. Our objective was to develop an approach to obtain intact antibody secreting cells from clinical samples of cryopreserved muscle biopsies.
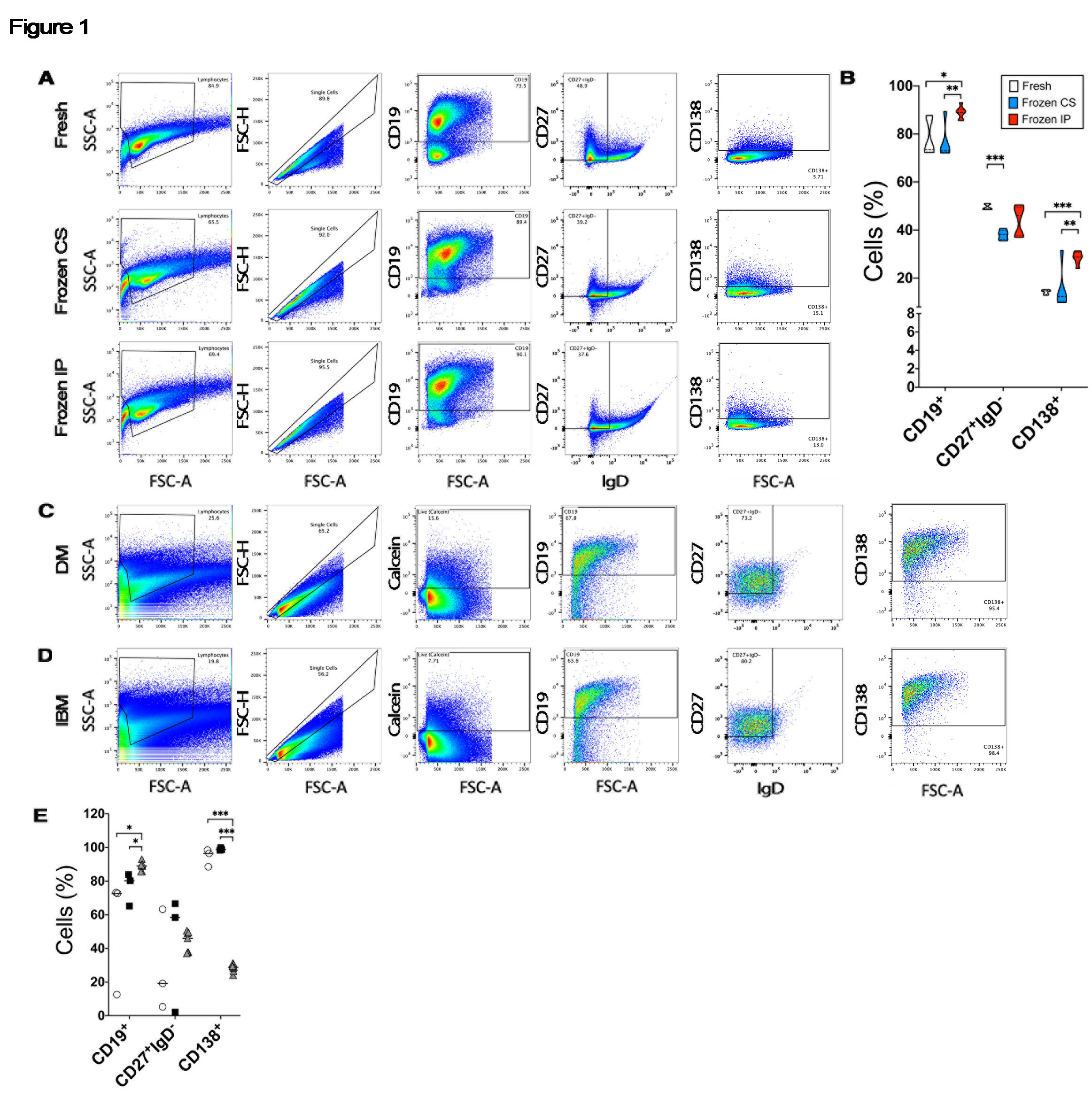
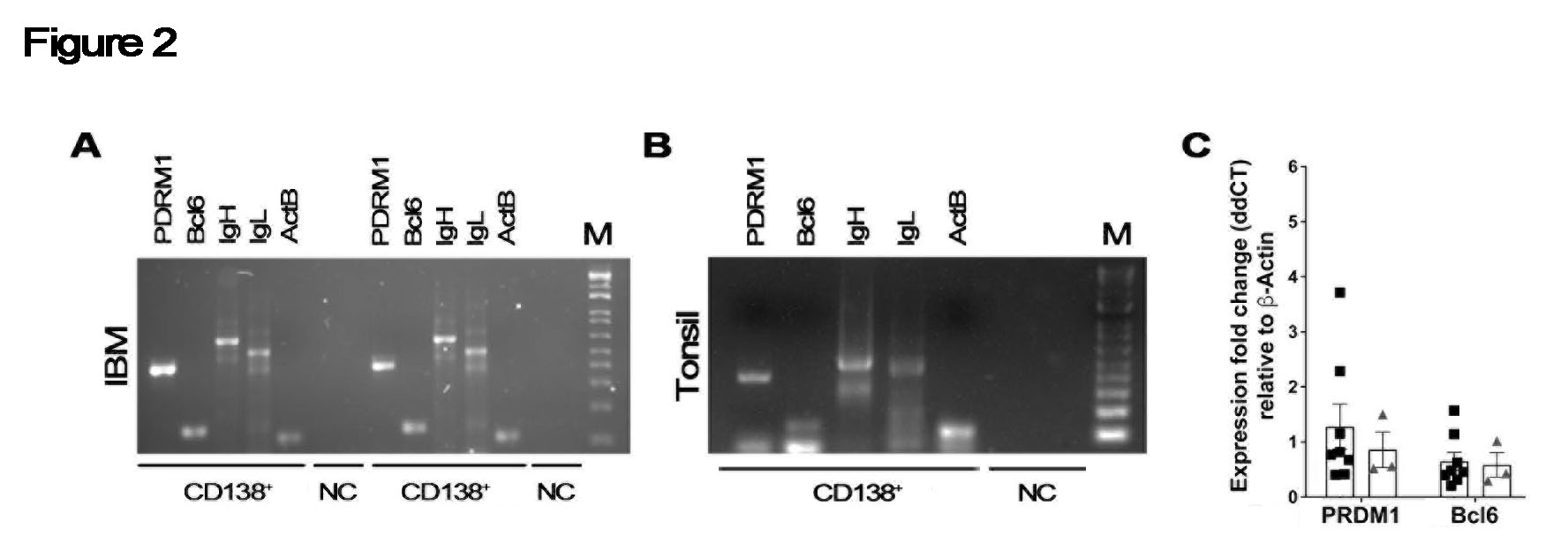
Methods: Recent publications described the ability to isolate intact cells from frozen biopsies of human lupus nephritis and rheumatoid arthritis. These relied on collecting material in HypoThermosol FRS (HT), followed by freezing using CryoStor CS10 (CS) medium (both STEMCELL Technologies, Cambridge, MA) – a method not currently used in clinical practice. The authors achieved non-inferior results compared to fresh tissue. We integrated the above approach with published methods of preparing non-human primate muscle and tested tissue processing, freezing, and storage methods. Paired deidentified 1 cm3 tonsil samples were subjected to variable collection media [HT or normal saline (NS)], freezing method [CS or liquid nitrogen-cooled isopentane (IP)], and digestion protocols. Flow cytometry was used to identify major antibody secreting cell subsets. Stored clinical DM and IBM samples were subjected to the final validated protocol and CD138+ cells were sorted into 96-well plates. PCR was performed to confirm expression of heavy and light chain and PRDM1 and Bcl6.
Results: We recovered similar proportions of CD19+ cells and downstream CD27+IgD– and CD138+ cell subsets by flow cytometry from tonsil collected with NS (Fig. 1A). Quantitation of our data showed that IP freezing was non-inferior to CS with statistically greater recovery of CD19+ (p=0.002) and CD138+ (p< 0.001) cells compared to fresh tissue (Fig. 1B). IP freezing outperformed CS conditions for CD19+ (p=0.001) and CD27+IgD– cells (p=0.002). Substitution of HT for NS did not alter results (data not shown).
Applying the digestion protocol to 6 muscle biopsies (3 DM, 3 IBM) showed similar proportions of live CD19+ B cells and downstream populations (Fig. 1C and D). All samples had recoverable cells despite an average sample age of 6.5 years. A quantitative comparison revealed no statistically significant differences in isolated cell populations between DM (open circle) and IBM (black square), while both disorders exhibited significantly greater proportions of CD138+ cells compared to tonsil (p < 0.001) (Fig. 1E). When subjected to PCR, examined single cells from IBM expressed immunoglobulin heavy and light chains (Fig. 2A). Levels of PDRM1 and Bcl6 from IBM (black square) were similar to tonsil controls (grey triangle) (Fig. 2B).
Conclusion: By making it feasible to use stored samples of frozen muscle tissue, the diagnosis and histology of which are known, our approach serves to make a large impact on human translational research of myositis. A major benefit is that the derived muscle digestion protocol does not require specialized collection or freezing media and is compatible with standard clinical samples. We plan to use this approach in the study of antibody repertoire and in situ immune response in IIM.
To cite this abstract in AMA style:
Zlobin A, Pytel P, Liarski V. Flow Cytometry and Sorting of Single Antibody Secreting Cells from Frozen Muscle Tissue [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/flow-cytometry-and-sorting-of-single-antibody-secreting-cells-from-frozen-muscle-tissue/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/flow-cytometry-and-sorting-of-single-antibody-secreting-cells-from-frozen-muscle-tissue/