Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Malondialdehyde (MDA) is produced in response to oxidative stress and is associated with inflammation and disease pathogenesis. MDA can break down and form acetaldehyde (AA). Together MDA and AA can non-enzymatically combine to create a stable protein adduct termed malondialdehyde-acetaldehyde adduct (MAA), which is strongly immunogenic, even in the absence adjuvant. Our group showed that MAA modified proteins are present in Rheumatoid Arthritis (RA) synovium and co-localize with citrullinated (CIT) antigens known to be strongly associated with the pathogenesis of RA. RA synovium is characterized by a combination of pro-inflammatory and pro-fibrotic signals secreted by immune cells and resident human fibroblast-like synoviocytes (HFLS). The purpose of this study is to evaluate pro-inflammatory and pro-fibrotic signals released by HFLS from patients with RA (HFLS-RA) vs. HFLS from patients with osteoarthritis (HFLS-OA) when stimulated with an extracellular matrix protein fibrinogen (Fib) modified with MAA, CIT, or the combination of MAA-CIT.
Methods: The HFLS-RA and HFLS-OA cell lines were cultured for 24 hours in the presence of 25 µg/mL of; unmodified Fib or Fib modified with MAA, CIT, or MAA-CIT. Supernatants were collected and analyzed by ELISA using the Meso Scale Diagnostics (MSD) platform for the release of pro-inflammatory and pro-fibrotic markers.
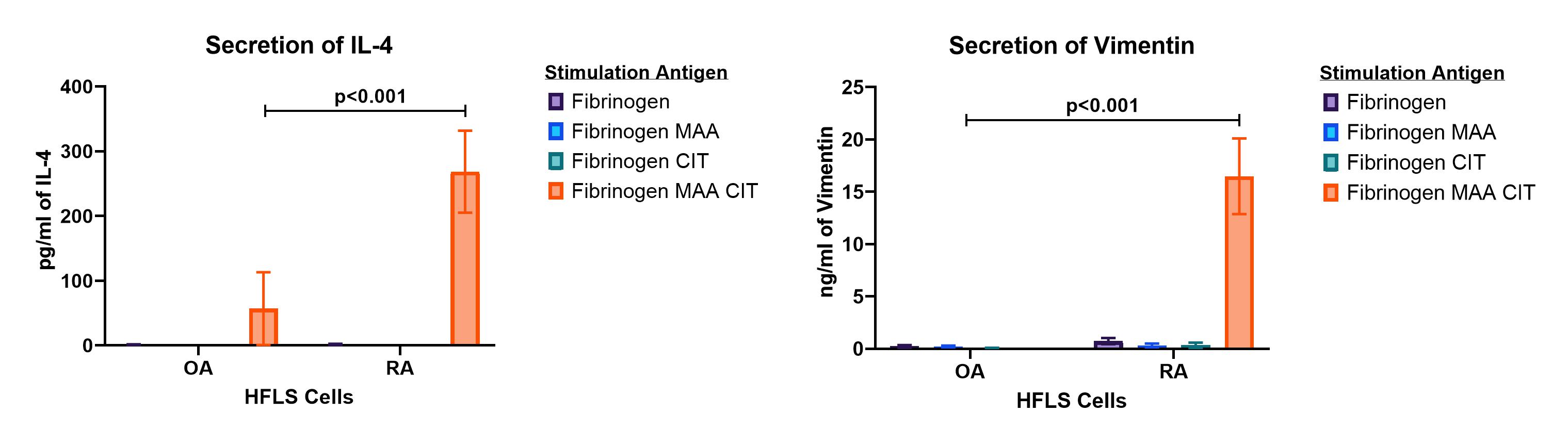
Results: Compared to HFLS-OA cells, HFLS-RA cells stimulated with Fib-MAA-CIT (Figures 1 and 2) significantly increased the secretion of vimentin and IL-4 (pro-fibrotic markers), and IL-6, IL-8, and MCP-1 (pro-inflammatory markers). HFLS-RA cells stimulated with Fib-CIT showed significantly increased levels of only the pro-inflammatory markers; IL-6 and MCP-1 (Figure 2) as compared to HFLS-OA cells. Additionally, HFLS-RA cells stimulated with Fib-MAA showed significantly increased secretions of the pro-inflammatory marker IL-8 (Figure 2B) compared to HFLS-OA cells.
Conclusion: These studies demonstrate that HFLS-RA cells secreted significantly increased pro-inflammatory and/or pro-fibrotic markers when compared to HFLS-OA cells depending on whether fibrinogen was modified with MAA, CIT or MAA-CIT. Additionally, these data also suggest that the HFLS-RA cells developed unique cellular responses compared to OA cells, making them useful in understanding how these modified proteins affect HFLS cells. Thus, further studies will evaluate the effects of other extracellular proteins that have been implicated in the pathogenesis of RA, and modified with MAA, CIT or MAA-CIT on the expression of pro-inflammatory and pro-fibrotic markers.
To cite this abstract in AMA style:
Wordekemper B, Aripova N, Duryee M, Daubach E, England B, O'Dell J, Mikuls T, Thiele G. Fibrinogen Modified with Malondialdehyde-Acetaldehyde Adduct (MAA) And/or Citrulline (CIT) Induces Unique Cellular Responses in Human RA Synoviocytes [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/fibrinogen-modified-with-malondialdehyde-acetaldehyde-adduct-maa-and-or-citrulline-cit-induces-unique-cellular-responses-in-human-ra-synoviocytes/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/fibrinogen-modified-with-malondialdehyde-acetaldehyde-adduct-maa-and-or-citrulline-cit-induces-unique-cellular-responses-in-human-ra-synoviocytes/