Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: There are limited non-surgical options for patients with severe pain due to knee osteoarthritis (KOA).Using geniculate artery embolization (GAE) to infarct hypervascular synovium, a known correlate of pain, has been proposed as a potential novel therapy. However, GAE is invasive and requires a lower extremity angiogram, which may limit its acceptability. Understanding patient-centered acceptability of new therapies is crucial to gauge potential uptake and to estimate enrollment for randomized controlled trials (RCTs). The goal of this study is to evaluate whether GAE is acceptable to patients with painful KOA, and to explore the feasibility of performing an RCT of GAE.
Methods: Patients >50 years with a KOA ICD-10 code (M17.0, M17.1, M17.9) were identified from practices of rheumatologists and physiatrists at a musculoskeletal specialty hospital. Exclusion criteria included Kellgren and Lawrence grade IV, systemic rheumatic disease, prior surgery to the index knee, contemplating arthroplasty, severe comorbidities and a KOOS pain subscale >70. Subjects used a remote viewing platform to watch a 20-minute PowerPoint presentation, which described GAE, including its risks and benefits, and presented a hypothetical sham-controlled RCT (i.e. angiogram with GAE vs. angiogram alone). A trained research assistant conducted the call and was available to answer questions. After completion, the subject was asked whether they would find GAE acceptable in three scenarios: 1) routine clinical care 2) an RCT where 50% of subjects would receive an angiogram but no GAE 3) the same RCT but with the option for the angiogram-only group to receive GAE later.
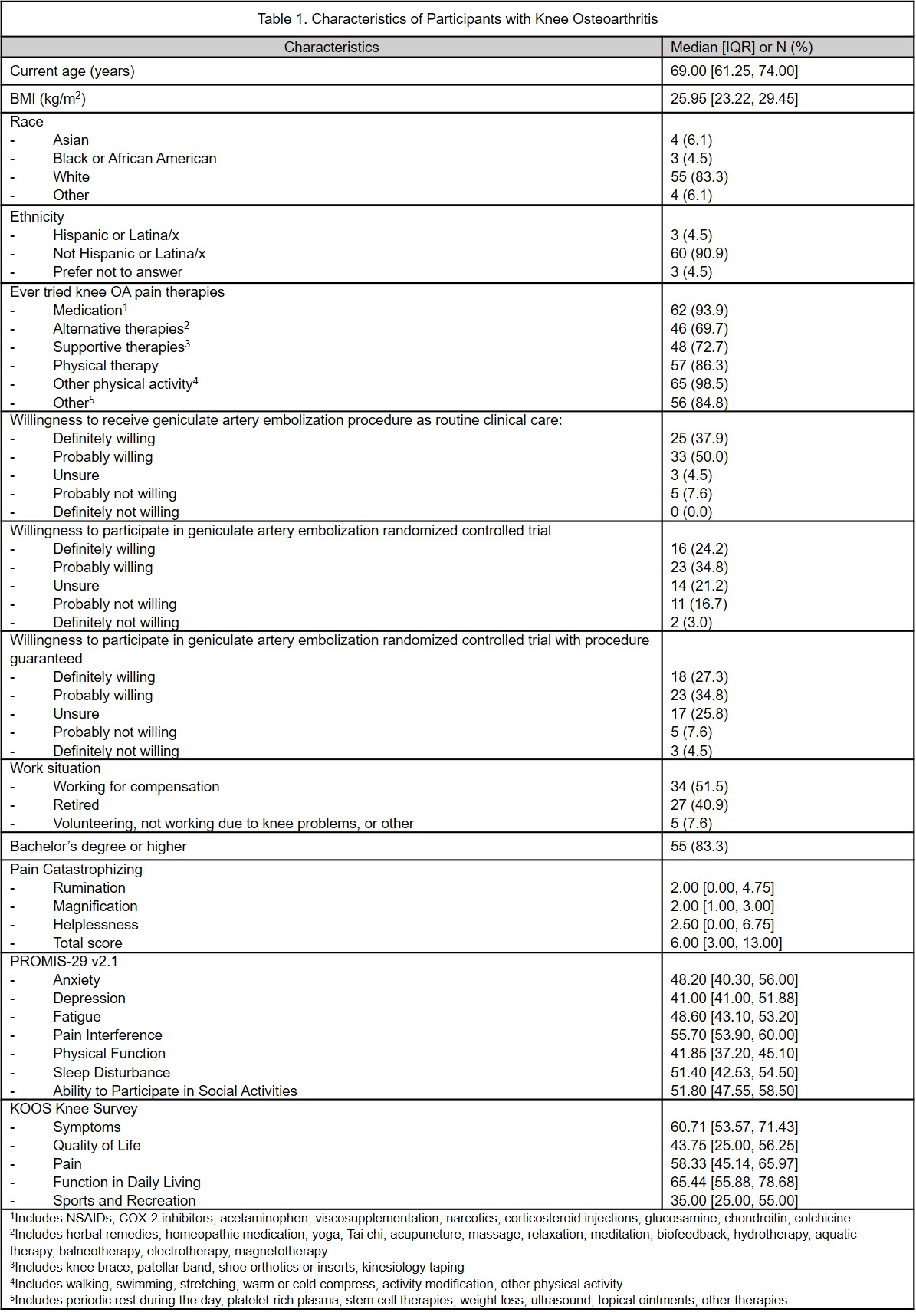
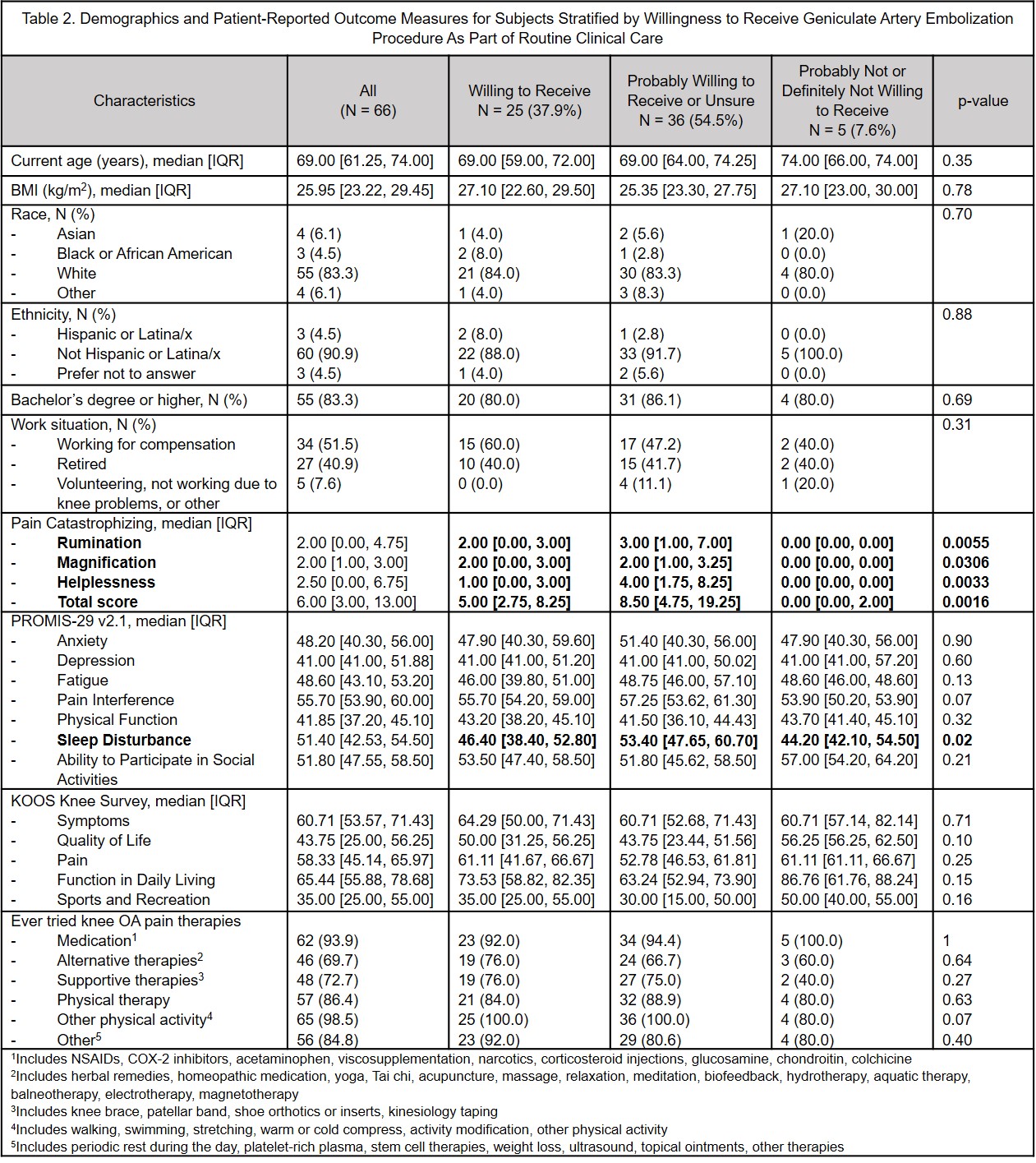
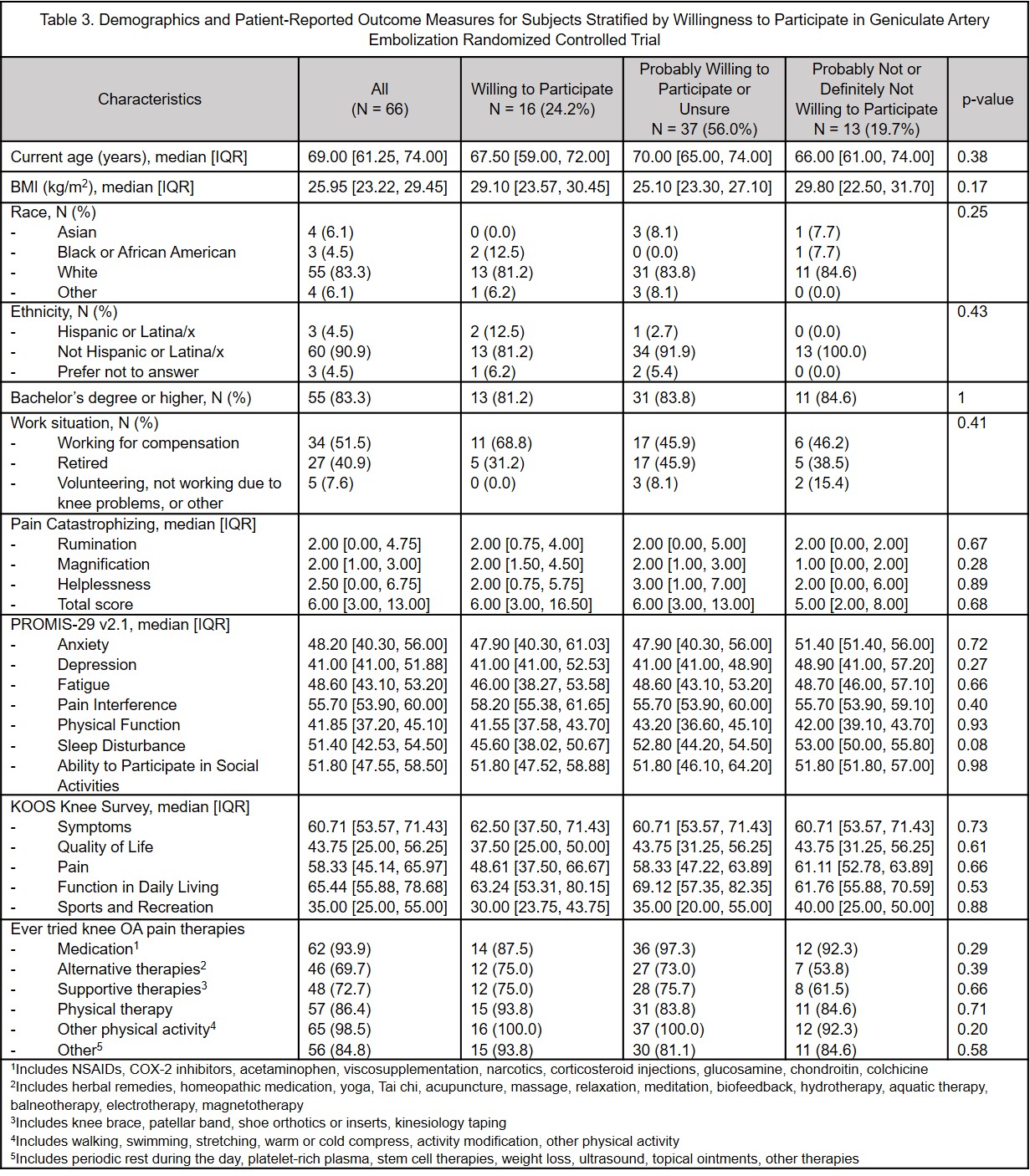
Results: 66 subjects enrolled, median age 69 [IQR: 61-74] years, 83% White, 4.5% Hispanic/LatinX, median BMI 26 [IQR: 23.2-29.5] kg/m2. Overall, 37.9% would be definitely willing and 50% probably willing to have GAE as a routine clinical intervention; 24.2% would be definitely willing and 34.8% probably willing to participate in a sham controlled RCT; this increased to 27.3% and remained 34.8%, respectively, if GAE was offered to the sham control group later. Those definitely/probably unwilling to have GAE as routine care had lower catastrophizing scores, but they were not clinically meaningfully different. There were no other clinically or statistically significant differences in age, race, ethnicity, BMI, KOOS or PROMIS-29 between those willing or not willing to have GAE in any of the three scenarios.
Conclusion: Approximately 40% of representative patients with painful KOA would be definitely willing to undergo GAE and 24.2% would be definitely willing to participate in a sham-controlled RCT. Guaranteeing access to GAE as part of an RCT may only minimally improve recruitment. These data can inform trial planning. The large proportion of subjects willing to undergo GAE underscores the unmet therapeutic need in this patient population, and similar acceptability across different demographic strata suggests GAE could benefit a wide range of patients with KOA.
To cite this abstract in AMA style:
Smole A, Swett B, Zhao A, Shea Y, Gottesman S, Sun D, Westrich G, Alexiades M, Gonzalez Della Valle A, Kishore S, Mandl L. Feasibility and Acceptability of Geniculate Artery Embolization for the Treatment of Painful Knee Osteoarthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/feasibility-and-acceptability-of-geniculate-artery-embolization-for-the-treatment-of-painful-knee-osteoarthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/feasibility-and-acceptability-of-geniculate-artery-embolization-for-the-treatment-of-painful-knee-osteoarthritis/