Session Information
Date: Tuesday, November 9, 2021
Title: RA – Treatments Poster III: RA Treatments & Their Safety (1674–1710)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Low-dose glucocorticoid (GC) therapy is widely used in rheumatoid arthritis (RA) but the balance of benefit and harm is still unclear. We studied the effects of prednisolone (5 mg/day, 2 years) added to standard of care in patients aged≥65 with active RA (1988 or 2010 criteria).
Methods: Pragmatic double-blind placebo-controlled randomized trial; all co-treatments and changes therein were allowed during the trial except long-term open label GC; Ca/D supplementation was advised in all patients. Minimal exclusion criteria were tailored to seniors.
Benefit outcomes: disease activity (DAS28) and joint damage (Sharp/van der Heijde).
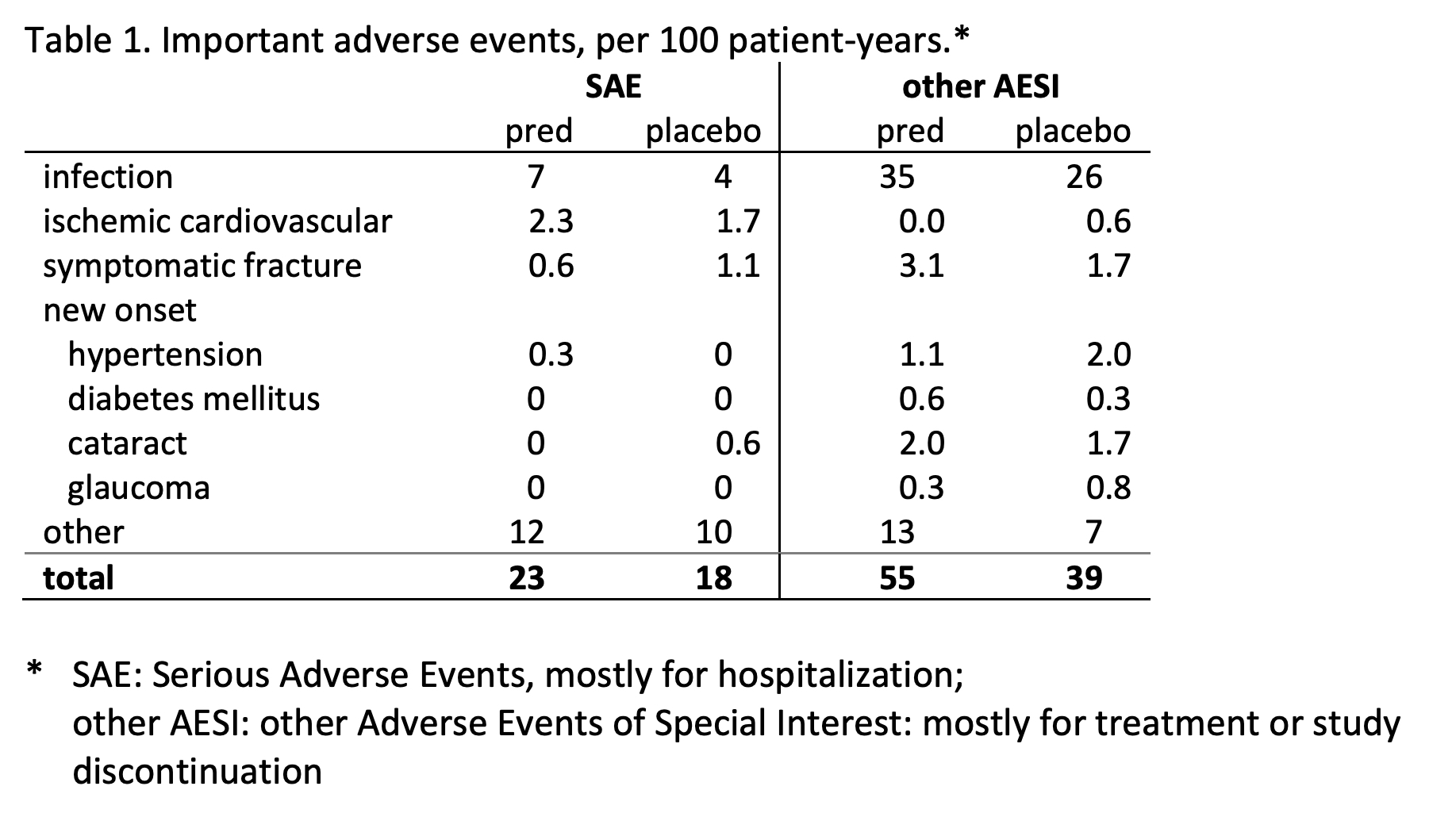
Harm outcome: proportion of patients with ≥1 adverse event (AE) of special interest: includes serious events, GC-specific events and those causing study discontinuation.
Analysis: longitudinal models, one-sided testing and 95% confidence limits (95%CL).
Results: We randomized 451 patients with established, impactful RA and mean 2.1 comorbidities: mean age 72 (max 88) years, 70% female, RA duration 11 years; 67% were RF+, 56% ACPA+, 96% had joint damage on radiographs; mean DAS28 4.5. 79% were on disease-modifying treatment, including 14% on biologics. 63% prednisolone vs 61% placebo patients completed the trial. Discontinuations were for AE (14%), active disease (4%), and for other (incl. covid-related) reasons (20%) in both groups; mean time in study 19 months.
Prednisolone resulted in more benefit and harm than placebo. Disease activity rapidly declined in the first 3 months and stabilized after 1 year (Figure 1), and was lower in prednisolone patients (adjusted mean difference in DAS28 over 2 years: 0.37, 95%CL 0.23, p< 0.0001). In 331 patients adherent to protocol on stable treatment (‘pure treatment comparison’) the mean difference in DAS28 after 3 months was 0.62 (95%CL 0.44). Significant time-treatment interaction in secondary analyses suggested a decrease in contrast after the first year; this was most likely caused by significantly more changes in DMARD treatment favoring the placebo group. Joint damage progression over 2 years was 1.7 point lower in the prednisolone group (95%CL 0.7, p=0.003).
60% prednisolone vs 49% placebo patients experienced the harm outcome: 60 vs 49%, adjusted RR 1.24, 95%CL 1.04, p=0.02; largest contrast in (mostly non-severe) infections (Table 1). Other GC-specific events were rare. In both groups, AE clustered in a subgroup of patients (Figure 2).
Conclusion: Add-on low dose prednisolone has powerful long-term effects in established RA, with a tradeoff of 24% relative (11% absolute) increase in the number of patients with adverse events, mostly non-severe; this suggests a favorable balance of benefit and harm.
This trial was funded by the European Union’s Horizon 2020 research and innovation program under grant agreement No. 634886.
 Figure 1. Change in DAS28 estimated in primary model, model with time-treatment interactions, and as observed, with numbers of patients. The grey area depicts the one-sided 95%confidence bound for the difference at each time point. Error bars depict one half of the two-sided 95%CI for the group means.
Figure 1. Change in DAS28 estimated in primary model, model with time-treatment interactions, and as observed, with numbers of patients. The grey area depicts the one-sided 95%confidence bound for the difference at each time point. Error bars depict one half of the two-sided 95%CI for the group means.
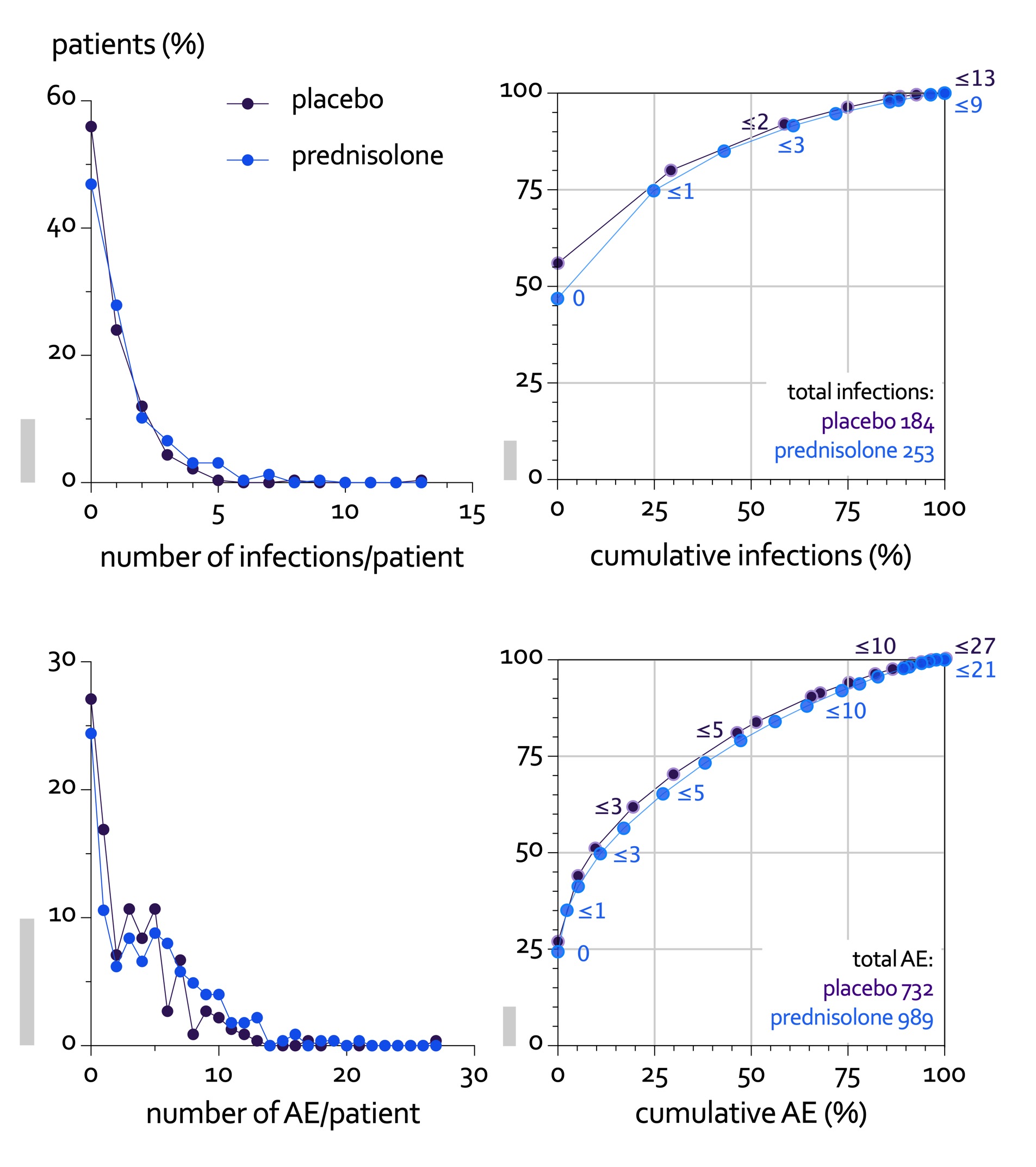 Figure 2. Patients with multiple adverse events (AE) have a major impact on the total number of events in both treatment groups: infection events in top panels, all AE in bottom panels. Left panels show the distribution of patients by number of events/patient. This shows most patients experience no or only a few events. Right panels plot bivariate cumulative distributions of patients by AE. The numbers next to the series indicate the maximum number of events of the population at that point. The right panels show the impact of multiple events: for example, 75% of patients (with 0 or 1 infection) contribute only 25% of all infections (top right panel); read in reverse, 50% of patients (with 2 resp. 3 or more events) contribute almost 90% of all AE (bottom right panel). To facilitate interpretation, grey vertical bars indicate 10% on the vertical scale.
Figure 2. Patients with multiple adverse events (AE) have a major impact on the total number of events in both treatment groups: infection events in top panels, all AE in bottom panels. Left panels show the distribution of patients by number of events/patient. This shows most patients experience no or only a few events. Right panels plot bivariate cumulative distributions of patients by AE. The numbers next to the series indicate the maximum number of events of the population at that point. The right panels show the impact of multiple events: for example, 75% of patients (with 0 or 1 infection) contribute only 25% of all infections (top right panel); read in reverse, 50% of patients (with 2 resp. 3 or more events) contribute almost 90% of all AE (bottom right panel). To facilitate interpretation, grey vertical bars indicate 10% on the vertical scale.
To cite this abstract in AMA style:
Boers M, Hartman L, Opris-Belinski D, Bos R, Kok M, Pereira da Silva J, Griep E, Klaasen R, Allaart C, Baudoin P, Raterman H, Szekanecz Z, Buttgereit F, Masaryk P, Klausch L, Paolino S, Schilder A, Lems W, Cutolo M. Favorable Balance of Benefit and Harm of Long-Term, Low Dose Prednisolone Added to Standard Treatment in Rheumatoid Arthritis Patients Aged 65+: The Pragmatic, Multicenter, Placebo-Controlled GLORIA Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/favorable-balance-of-benefit-and-harm-of-long-term-low-dose-prednisolone-added-to-standard-treatment-in-rheumatoid-arthritis-patients-aged-65-the-pragmatic-multicenter-placebo-controlled-gloria-t/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/favorable-balance-of-benefit-and-harm-of-long-term-low-dose-prednisolone-added-to-standard-treatment-in-rheumatoid-arthritis-patients-aged-65-the-pragmatic-multicenter-placebo-controlled-gloria-t/