Session Information
Date: Monday, November 8, 2021
Title: Abstracts: Systemic Sclerosis & Related Disorders – Basic Science (1434–1437)
Session Type: Abstract Session
Session Time: 11:15AM-11:30AM
Background/Purpose: Early loss of skin-associated adipose tissue and concomitant replacement by extracellular matrix is a hallmark of systemic sclerosis (SSc). However, the contribution of adipose tissue to SSc pathogenesis remains poorly understood. In murine models of skin fibrosis, lipid-laden adipocytes acquire features of migratory myofibroblasts.1 This adipocyte-to-myofibroblast transition offers up a promising suggests a mechanism for direct contribution of skin-associated adipose tissue to the early SSc skin phenotype. The developmental gene sine oculis homeobox homolog 1 (SIX1) is known to regulate cell fate and transdifferentiation in disease. We hypothesize that elevated SIX1 promotes adipocyte-to-myofibroblast transition, thus contributing to loss of adipose tissue and progression of fibrosis in SSc.
Methods: Skin SIX1 expression levels were identified by RNA sequencing and DNA microarray in the PRESS and GENISOS cohorts, respectively. All patients satisfied ACR classification criteria for SSc. Clinical correlations between SIX1 were assessed by Spearman’s rank-order and Pearson’s correlation for continuous variables. Cellular localization of SIX1 was confirmed in patient and mouse skin samples by single molecule in situ hybridization. Ubiquitous genetic deletion of Six1 in eight-week-old mice was achieved using the tamoxifen-inducible Cre recombinase-loxp system. Immunofluorescent staining of collagen 6 (the primary collagen in fat tissue) was quantified as percent positive signal within the dermal adipose.
Results: SIX1 was upregulated in the affected skin of patients in the GENISOS and PRESS cohorts (Fig 1A & 1B, respectively) at the time of enrollment. Baseline SIX1 expression positively correlated with baseline modified Rodnan Skin Score (r = 0.37, p< 0.0001) and local skin score (r = 0.38, p< 0.0001). In dcSSc patients, SIX1 negatively correlated with disease duration at the time of enrollment (r= -0.28, p=0.022). SIX1 strongly correlated with a subcutaneous adipose gene expression signature in two independent cohorts and lipolysis-associated molecular pathways, supporting a driving role of SIX1 in adipocyte dysfunction. Six1 was overexpressed early in dermal adipocytes of the subcutaneous bleomycin murine model of skin fibrosis (Figure 2). When challenged with bleomycin, inducible Six1-deficient mice (Six1 KO) demonstrated lower fibrotic gene expression compared to control mice (Figure 3A). Six1 KO mice were protected against lipoatrophy (Figure 3B) and collagen 6 deposition within the dermal fat (Figure 3C).
Conclusion: We concluded that SIX1 is elevated in skin-associated fat of SSc patients, and enriched in early diffuse disease. Higher SIX1 expression was associated with worsened skin fibrosis in baseline samples. Inhibiting SIX1 can protect against atrophy of dermal fat and increased fibrotic responses in models of SSc-like skin fibrosis. Developmental genes that become activated during disease are ideal candidate targets for new therapeutics, as these genes are lowly expressed in healthy adult tissues, thus dramatically reducing the likelihood of off-target effects. Our findings support further investigation into SIX1 as a feasible drug molecular target for SSc.
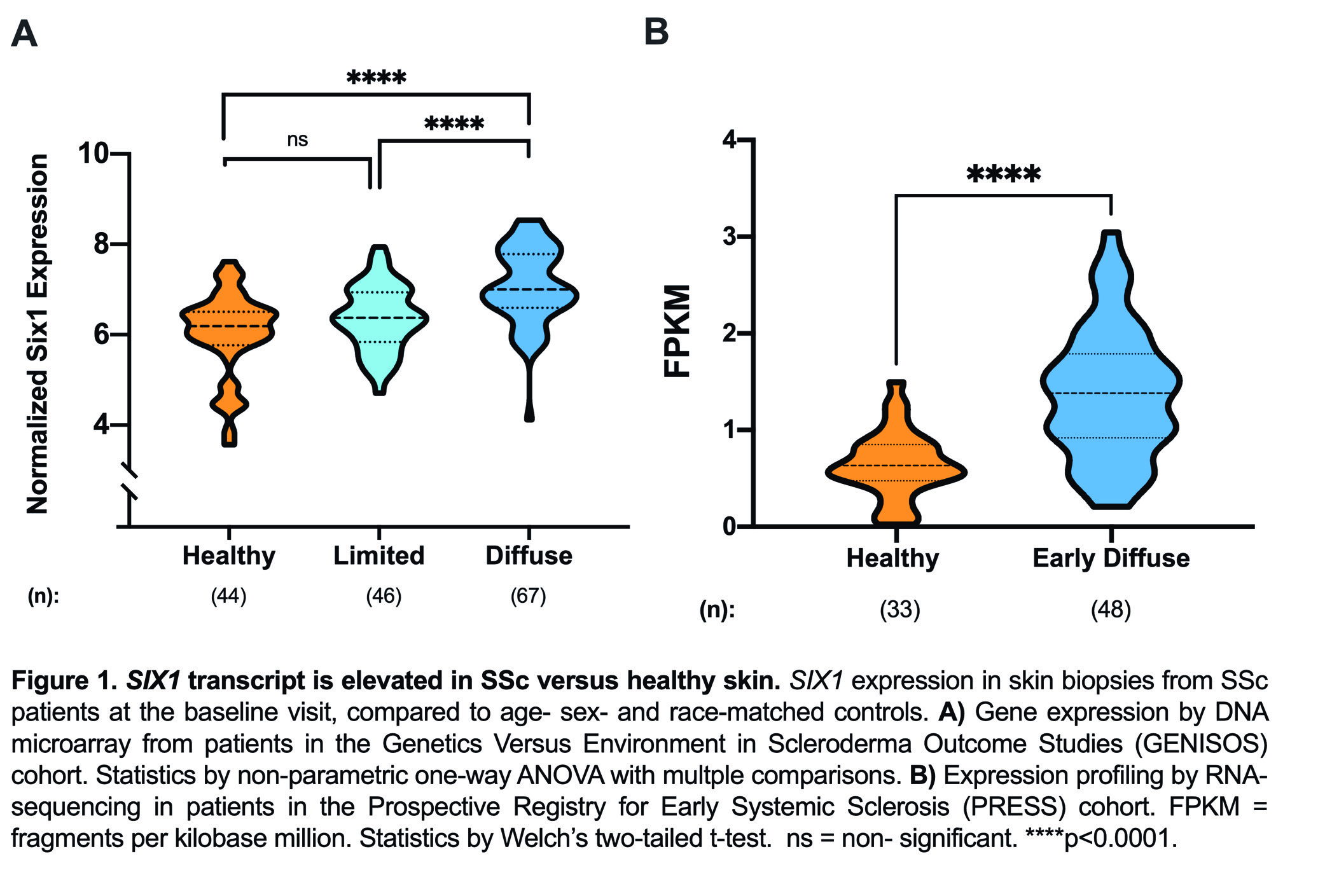 Figure 1. SIX1 transcript is elevated in SSc versus healthy skin.
Figure 1. SIX1 transcript is elevated in SSc versus healthy skin.
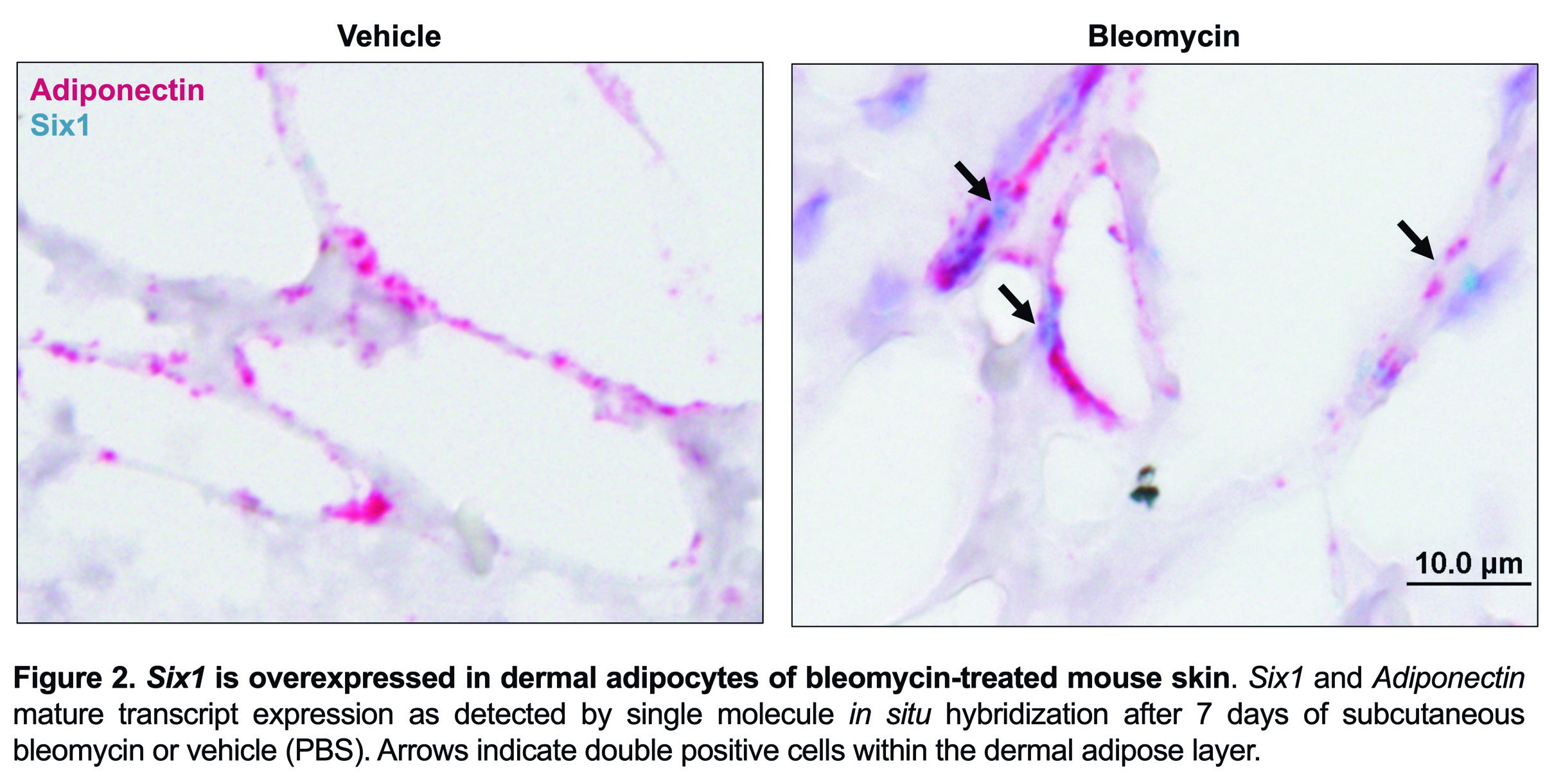 Figure 2. Six1 is overexpressed in dermal adipocytes of bleomycin-treated mouse skin.
Figure 2. Six1 is overexpressed in dermal adipocytes of bleomycin-treated mouse skin.
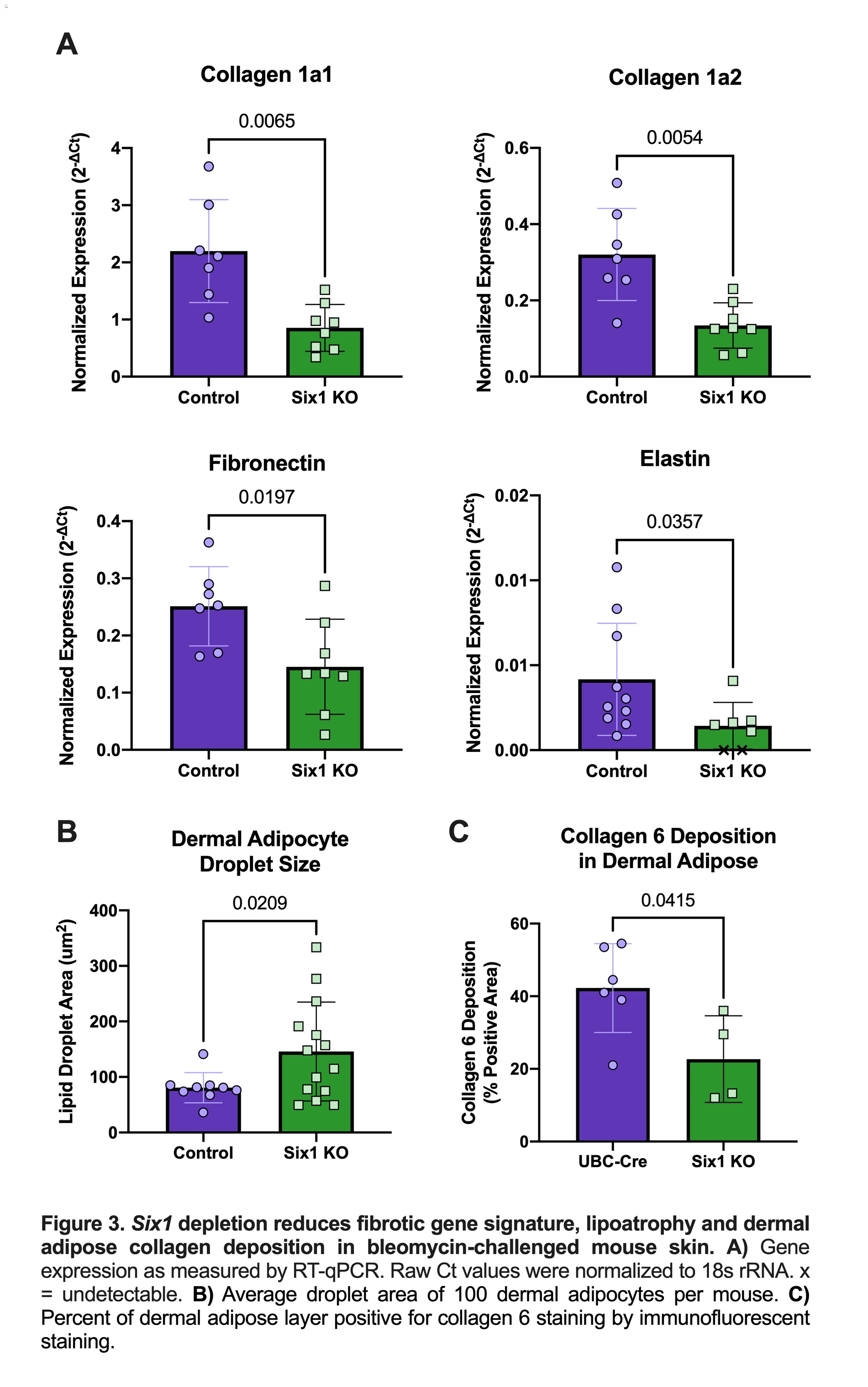 Figure 3. Six1 depletion reduces fibrotic gene signature, lipoatrophy and dermal adipose collagen deposition in bleomycin-challenged mouse skin.
Figure 3. Six1 depletion reduces fibrotic gene signature, lipoatrophy and dermal adipose collagen deposition in bleomycin-challenged mouse skin.
To cite this abstract in AMA style:
Wareing N, Skaug B, Wu M, Collum S, Wilson C, Revercomb L, Lyons M, Bi W, Mills T, Charles J, Assassi S, Karmouty-Quintana H. Fat and Fibrosis: A Novel Developmental Gene in Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/fat-and-fibrosis-a-novel-developmental-gene-in-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/fat-and-fibrosis-a-novel-developmental-gene-in-systemic-sclerosis/
