Session Information
Date: Sunday, November 8, 2020
Title: RA – Diagnosis, Manifestations, & Outcomes Poster III: Cardiopulmonary Aspects
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: A novel score for predicting 3-year risk for CVD events in RA patients combines age, four traditional CVD risk factors (diabetes, hypertension, smoking, history of high-risk CVD event), a personalized assessment of RA-related inflammation based on the multi-biomarker disease activity (MBDA) score and, individually, 3 of its 12 biomarkers, TNF-R1, MMP-3 and leptin (log-transformed). This score was developed and internally validated using patient data from the Medicare database. The purpose of this analysis was to externally validate the MBDA-based CVD risk prediction score in a younger cohort from the Symphony claims database.
Methods: A cohort of patients ≥18 years old with RA diagnosis from a rheumatologist and evidence of an RA-specific treatment, excluding patients with malignancy, past myocardial infarction (MI) or stroke, was created by a third party (Symphony) by matching medical and pharmaceutical claims and linking them to MBDA scores from a database of tests done for routine care. Medicare patients were excluded to avoid overlap with the internal validation cohort. Only the first MBDA test was used for each patient. The study endpoint was time from MBDA testing to first CVD event within a 3-year time horizon. CVD event was defined as MI or stroke, based on ICD-9 or ICD-10 diagnosis codes in hospital claims. Analyses focused on relative risk, not absolute risk, because CVD event data in Symphony may be incomplete. A univariate Cox proportional hazards regression model was fit with the MBDA-based CVD risk score as the sole predictor of time to CVD event to obtain a hazard ratio (HR) estimate (95% CI) and p-values from a likelihood ratio test (LRT). Sensitivity analyses determined HR for patient subgroups, with p-values determined for the interaction between subgroups and the MBDA-based CVD risk score. Using a multivariate Cox proportional hazard regression model, the MBDA-based CVD risk score was compared to a simpler model that included only age, sex, diabetes, hypertension, history of other CVD, smoking and CRP (log-transformed) for ability to predict time to a CVD event.
Results: 48,868 patients with 337 CVD events met eligibility criteria and had linked biomarker data. Mean age was 54.4 years. 81.7% were female (Table 1). Mean follow-up was 24.4 months. The MBDA-based CVD risk score (mean 3.3, IQR 2.8–3.8) was highly significant in univariate analysis, with HR = 3.99 (95% CI: 3.52-4.51, p = 4.4×10-95); i.e., for every 1-unit increase in risk score, the CVD event rate in this cohort was ~4 times as high. Similar results were seen in the subset of 44,379 patients < 65 years old, with HR=4.26 (95% CI: 3.53-5.14, p = 1.2×10-47). In sensitivity analyses, after adjusting for multiple comparisons, there were no significant differences between HR of complementary subgroups (Figure 1). The MBDA-based CVD risk score added significant prognostic information to a simpler, clinical model (HR=2.28 [95% CI: 1.69-3.08, p = 1.6×10-7] after accounting for all other factors).
Conclusion: The MBDA-based CVD risk prediction score has been externally validated in a cohort that is younger than and independent of the Medicare cohort used previously for test development and internal validation.
 Table 1. Cohort characteristics of RA patients with linked biomarker data and at risk for CVD events.
Table 1. Cohort characteristics of RA patients with linked biomarker data and at risk for CVD events.
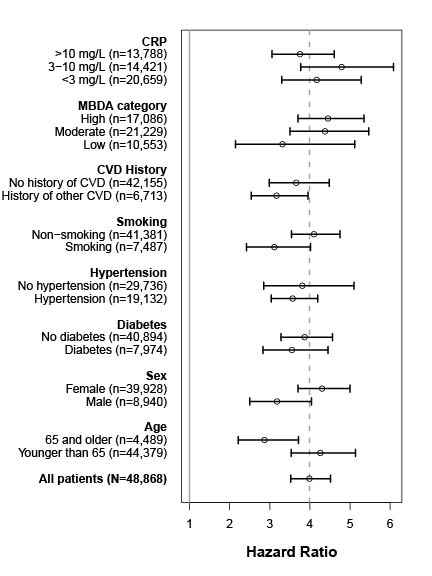 Figure 1. Hazard ratios for CVD event over 3 years in the external validation dataset (N=48,868). Vertical dashed line indicates the hazard ratio for all patients.
Figure 1. Hazard ratios for CVD event over 3 years in the external validation dataset (N=48,868). Vertical dashed line indicates the hazard ratio for all patients.
To cite this abstract in AMA style:
Curtis J, Sasso E, Hitraya E, Chin C, Bamford R, Ben-Shachar R, Gutin A, Flake D, Mabey B, Lanchbury J. External Validation of a Multi-biomarker-Based Cardiovascular Disease Risk Prediction Score for Rheumatoid Arthritis Patients [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/external-validation-of-a-multi-biomarker-based-cardiovascular-disease-risk-prediction-score-for-rheumatoid-arthritis-patients/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/external-validation-of-a-multi-biomarker-based-cardiovascular-disease-risk-prediction-score-for-rheumatoid-arthritis-patients/
