Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Intraarticular (IA) corticosteroids are generally considered safe and effective to treat osteoarthritis of the knee (OAK) but may cause hyperglycemia that may last for weeks and can be particularly severe for up to 72 hours after injection; this hyperglycemia can have adverse implications for patients who have comorbid type 2 diabetes mellitus (T2D). A phase 2 study of 33 patients with OAK and T2D (NCT02762370) found that there were minimal blood glucose disruptions when patients were administered extended-release triamcinolone acetonide (TA-ER) compared with immediate-release triamcinolone acetonide (TA-IR). The purpose of this post hoc analysis is to characterize the clinical meaningfulness and relevance of the results of the phase 2 study.
Methods: Patients who had T2D for ≥1 year, symptomatic OAK for ≥6 months (as defined by the American College of Rheumatology criteria for the Classification of OA of the Knee), and glycated hemoglobin ≥6.5% and < 9.0% were considered eligible and randomized to IA TA-ER or TA-IR. Before IA injection and throughout the subsequent 14 days, continuous glucose monitoring (CGM) provided an ambulatory glucose profile that summarized blood glucose levels (BGLs). Percentage of time in target range (70-180 mg/dL) and percentage of time above range (TAR; including >250 mg/dL) as defined by internationally recognized thresholds, changes from baseline in daily average BGL, and glycemic variability were compared between groups. Kaplan-Meier analysis was used to determine the time to reach 250 mg/dL and maximum BGL.
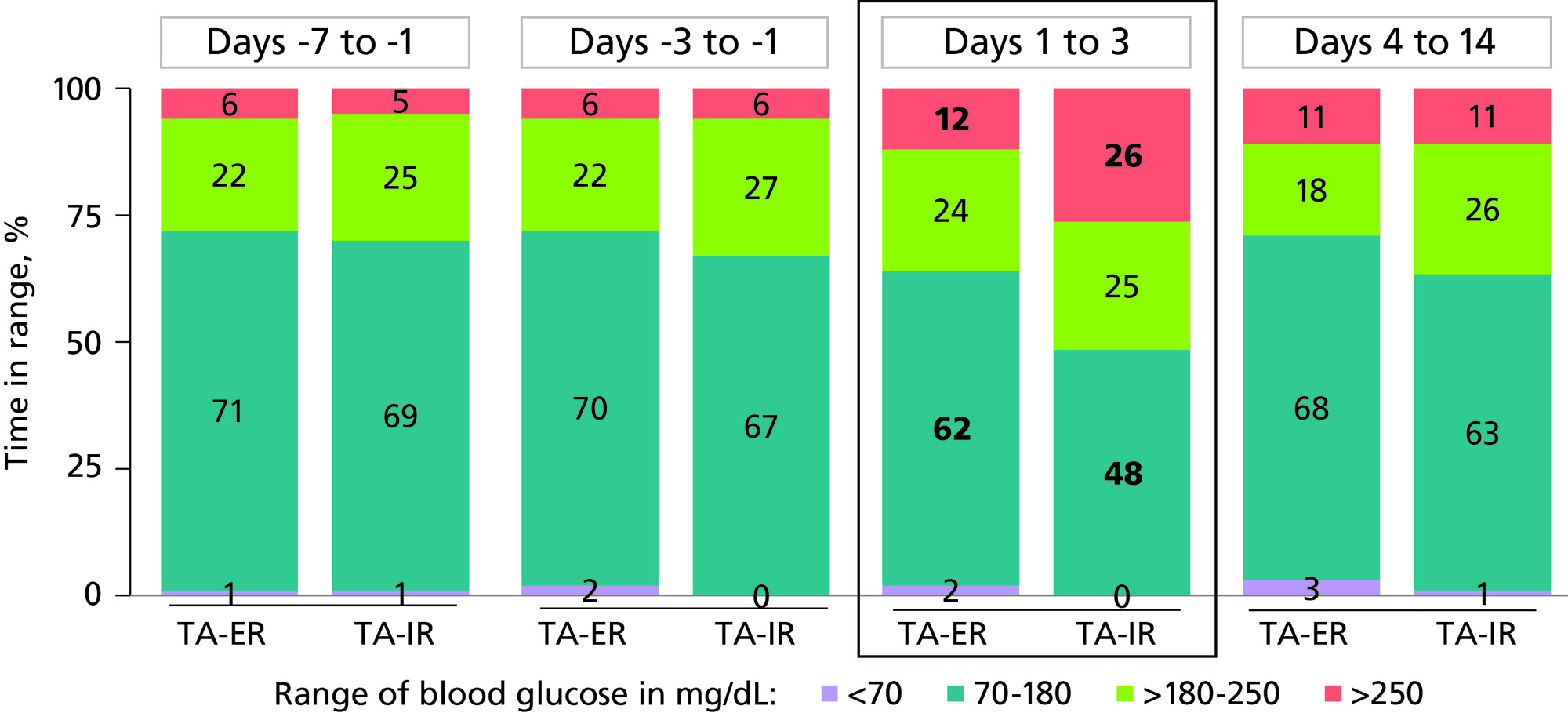
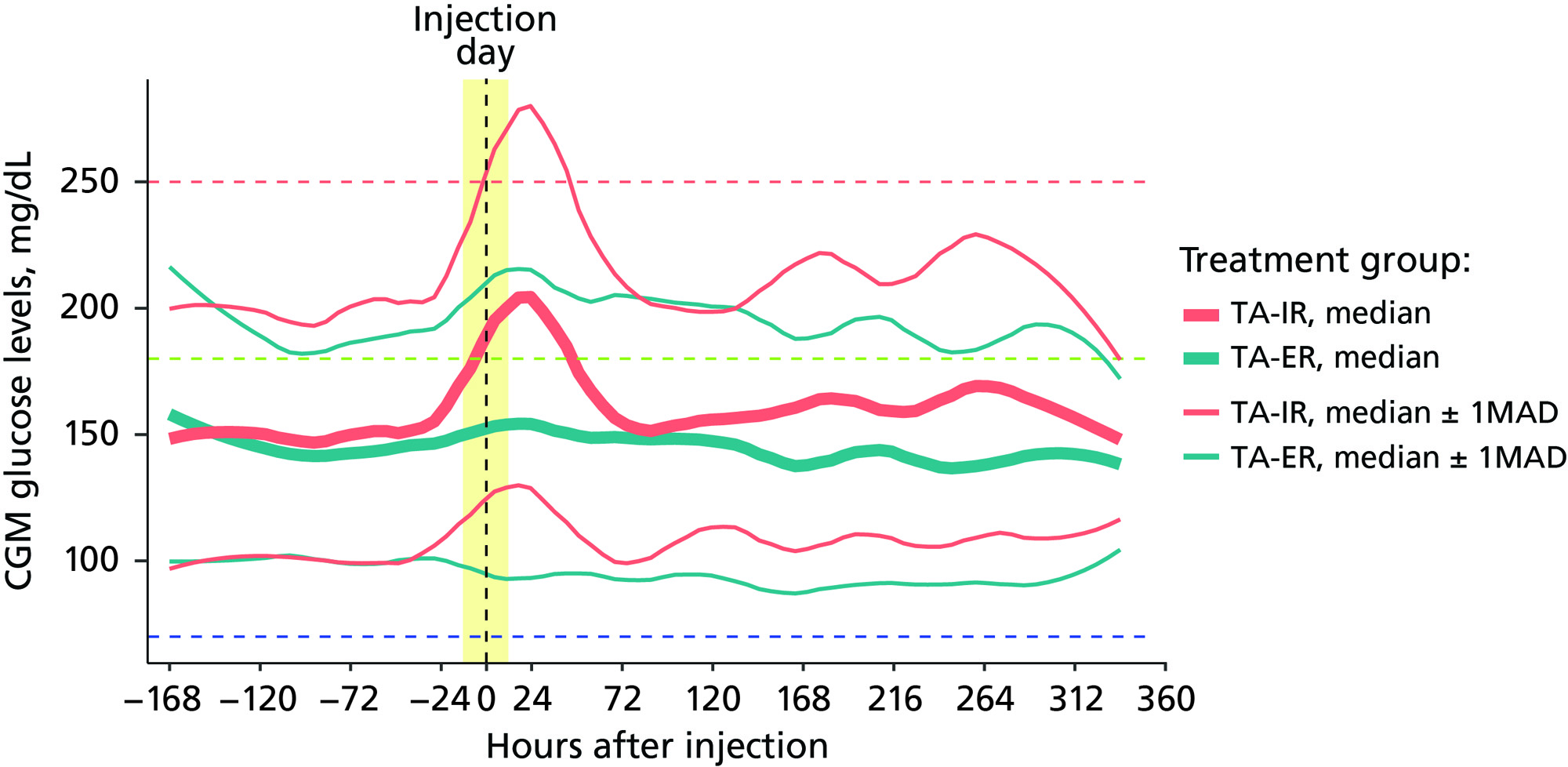
Results: Across days 1-3, IA TA-ER versus TA-IR resulted in an increased percentage of time in the target range (62% vs 48%; P=0.123) and a significant 2-fold lower percentage of TAR >250 mg/dL (12% vs 26%; P=0.047) (Figure 1). IA TA-ER had a smaller percentage of patients with maximum CGM BGL >250 mg/dL versus TA-IR (50% vs 93%). The ambulatory glucose profile analyses demonstrated more controlled BGLs and lower glucose spikes for patients receiving IA TA-ER compared with TA-IR (Figure 2). IA TA-ER had significantly higher time to maximum BGL versus TA-IR (34 vs 13 hours; P=0.007) and median time to 250 mg/dL (44 vs 6 hours; P=0.003).
Conclusion: This post hoc analysis suggests that IA TA-ER provides a clinically meaningful reduction in hyperglycemia versus TA-IR in patients with OAK and T2D. Glucose management is expected to improve with TA-ER compared with TA-IR because of the increased percentage of time in the target range. Lower and more controlled glucose spikes may lead to fewer short-term hyperglycemia-related adverse events for patients receiving TA-ER versus TA-IR. Overall, TA-IR may reduce adverse events and healthcare utilization while improving long-term glucose control and patient well-being, particularly with repeat injections.
To cite this abstract in AMA style:
Spitzer A, Rodbard H, Iqbal S, Nakazawa M, DiGiorgi M, Winston R. Extended-Release versus Immediate-Release Triamcinolone Acetonide for Osteoarthritis of the Knee with Comorbid Diabetes Type 2 Diabetes Mellitus: A Post Hoc Analysis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/extended-release-versus-immediate-release-triamcinolone-acetonide-for-osteoarthritis-of-the-knee-with-comorbid-diabetes-type-2-diabetes-mellitus-a-post-hoc-analysis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/extended-release-versus-immediate-release-triamcinolone-acetonide-for-osteoarthritis-of-the-knee-with-comorbid-diabetes-type-2-diabetes-mellitus-a-post-hoc-analysis/