Session Information
Date: Wednesday, November 15, 2023
Title: Abstracts: RA – Diagnosis, Manifestations, and Outcomes IV: Pre-RA & RA Diagnosis
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Production of ACPA following T and B cell interactions is a hallmark of ACPA+ RA. Recently identified peripheral helper T (Tph) cells have B cell-helper functions coupled with a migratory program targeting inflamed peripheral non-lymphoid tissues. Tph cells are expanded in the inflamed joint and blood of ACPA+ RA patients. However, it is not known whether immune dysregulation with an expansion of Tph cells occurs in a pre-disease stage of RA (i.e., ACPA+ at-risk individuals [ARI]).
Methods: We analyzed peripheral blood mononuclear cells (PBMC) from two independent and ethnically distinct cohorts to identify cell populations associated with serum ACPA positivity as well as eventual progression to classified RA. First, we employed mass cytometry with 38 markers in PBMCs from ARI, ACPA+ early RA patients, and healthy controls in a Japanese cohort (n = 17 in each group). Data were analyzed by computational analyses and biaxial gating. Next, the findings identified in the Japanese cohort were evaluated by flow cytometric analysis of PBMCs from a US-based cohort that included 11 healthy controls and 11 ACPA+ individuals who all subsequently developed RA (pre-RA), of which 9 had available post-RA diagnosis PBMC (post-RA), allowing for longitudinal analyses.
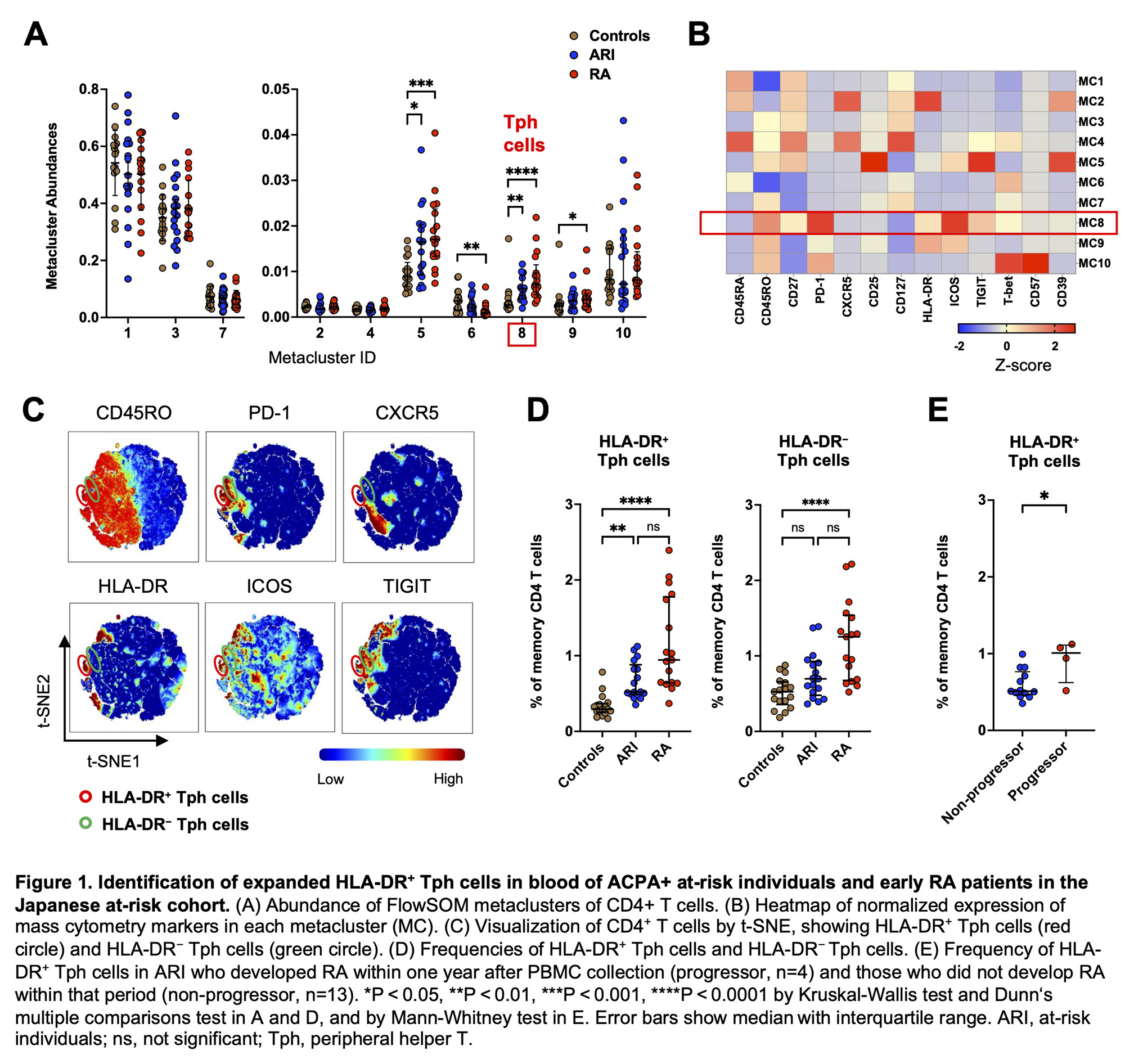
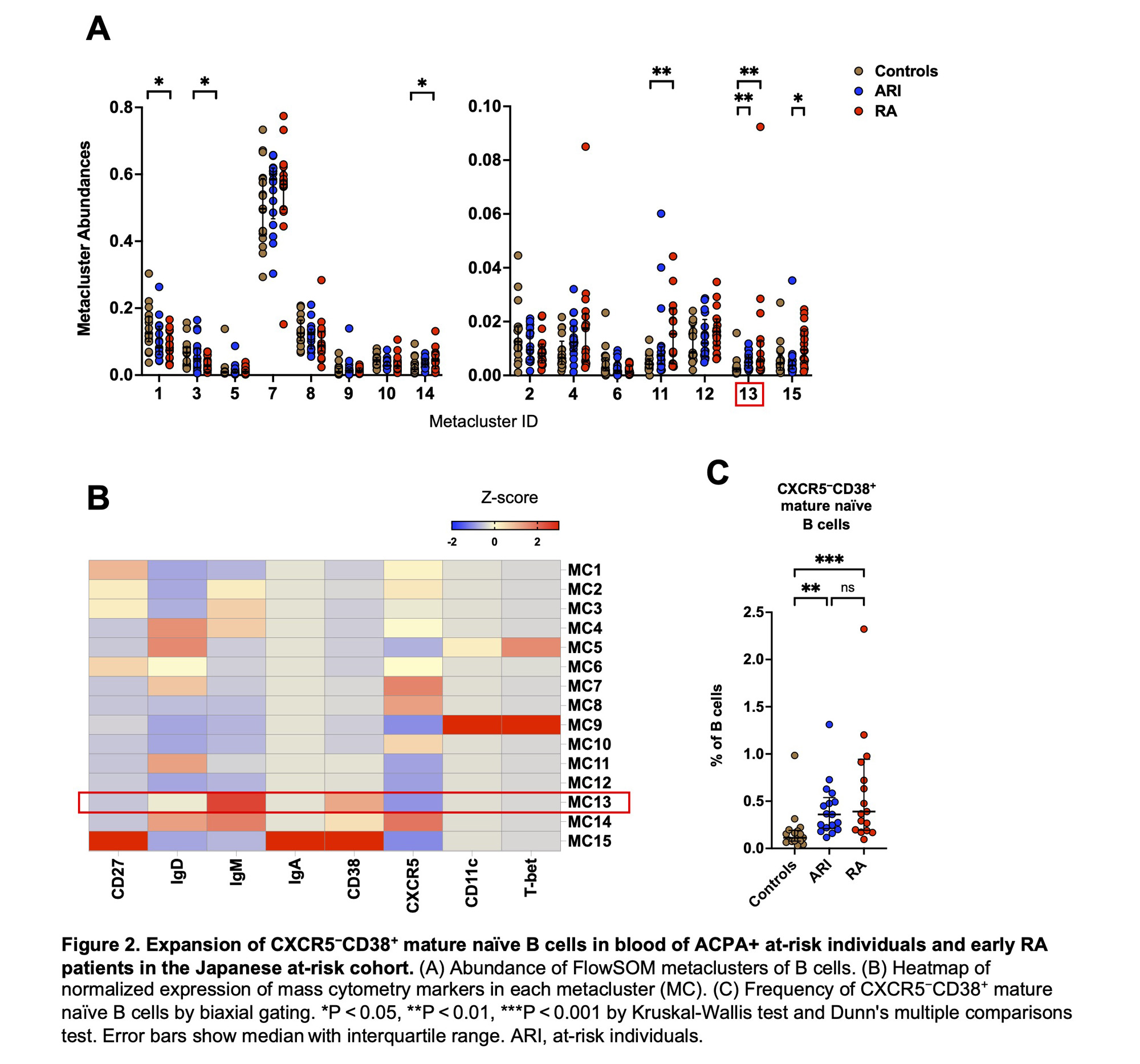
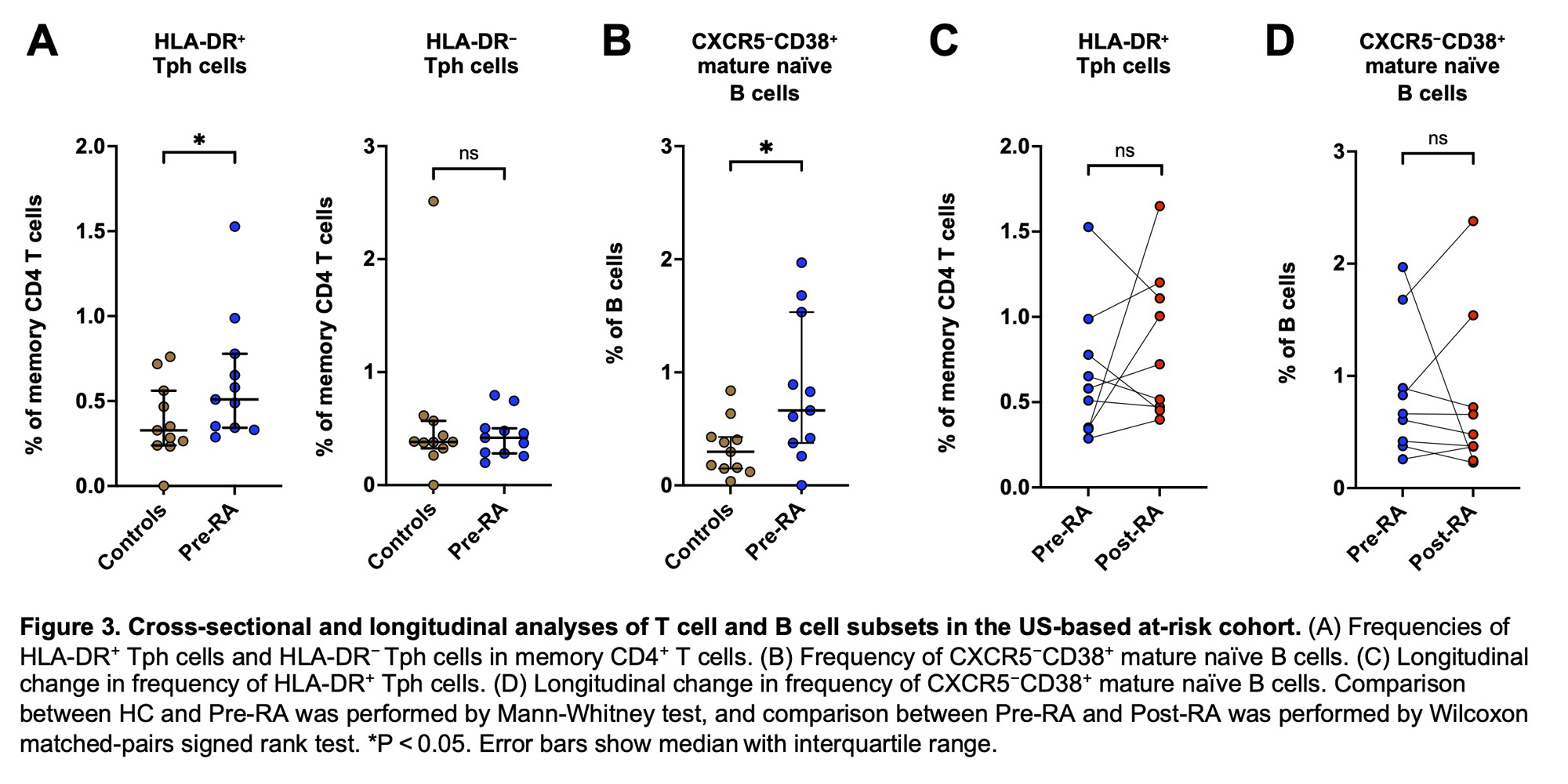
Results: In the Japanese cohort, FlowSOM analysis of CD4+ T cells identified 10 metaclusters (i.e., cell populations). Of those, two metaclusters were significantly increased in ARI and RA compared to controls (Figure 1A). One of these metaclusters showed a high level of PD-1 expression and lack of CXCR5 expression, consistent with Tph cells (Figure 1B). t-SNE clustering visualized Tph cells composed of two distinct subpopulations based on HLA-DR expression (Figure 1C). Biaxial gating revealed that HLA-DR+ Tph cells were significantly increased in ARI (p = 0.002) and RA (p < 0.001) compared to controls, while HLA-DR− Tph cells were increased only in RA (Figure 1D). In ARI, the frequency of HLA-DR+ Tph cells was higher in ‘progressors’ (i.e., ARI who developed RA within one year after PBMC collection) compared to non-progressors (p = 0.032, Figure 1E). The FlowSOM analysis of B cells identified 15 metaclusters, and one of these metaclusters was significantly expanded in ARI and RA compared to controls (Figure 2A). Heatmap indicated that this metacluster was IgM+IgD+CD38+CD27−CXCR5− cells (CXCR5−CD38+ mature naïve B cells) (Figure 2B). Biaxial gating confirmed their expansion in ARI and RA (p = 0.002, p < 0.001, respectively; Figure 2C). In the US-based cohort, HLA-DR+ Tph cells and CXCR5−CD38+ mature naïve B cells were also increased in pre-RA compared to controls (p = 0.047, p = 0.040, respectively; Figure 3AB). The frequencies of HLA-DR+ Tph cells and CXCR5−CD38+ mature naïve B cells did not significantly differ between pre-RA and post-RA visits (Figure 3CD).
Conclusion: The expansion of HLA-DR+ Tph cells and CXCR5−CD38+ mature naïve B cells in ACPA+ individuals, including those who develop classified RA, and the association of the former sub-type with ‘progressors’ supports a key role of these cells in ACPA positivity as well as a transition from pre-RA to classified RA. These cell sub-types may identify a mechanistic target for treatment and prevention in RA.
Disclosures: H. Takada: None; K. Demoruelle: Boehringer-Ingelheim, 5, Gilead, 5, Pfizer, 5; K. Deane: Bristol-Myers Squibb(BMS), 1, Gilead, 5, Janssen, 5, Werfen, 1, 12, Biomarker kits; S. Nakamura: None; Y. Katsumata: Asahi Kasei Pharma, 6, Astella, 6, AstraZeneca, 6, Chugai, 6, GlaxoSmithKlein(GSK), 6, Janssen, 6, Mitsubishi Tanabe Pharma Corporation, 6, Pfizer, 6; K. Ikari: AbbVie/Abbott, 6, Asahi Kasei, 6, Astellas Pharma, 6, Ayumi Parmaceutical, 6, Bristol-Myers Squibb(BMS), 6, Chugai Parmaceutical, 6, Eisai, 6, Eli Lilly, 6, Janssen, 6, Kaken Pharmaceutical, 6, Mitsubishi Tanabe Pharma, 6, Pfizer, 6, Takeda Pharmaceutical, 6, Teijin Pharma, 6, UCB, 6; J. Buckner: Bristol-Myers Squibb(BMS), 2, gentibio, 1, 10, 11, hotspot therapeutics, 2, Janssen, 2; W. Robinson: None; J. Seifert: None; M. Feser: None; L. Moss: None; J. Norri: None; M. Harigai: Astellas Pharma, 6, AstraZeneca, 6, GlaxoSmithKlein(GSK), 6, 12, Post marketing surveillence, Novartis, 5; E. Hsieh: None; M. Holer: None; Y. Okamoto: None.
To cite this abstract in AMA style:
Takada H, Demoruelle K, Deane K, Nakamura S, Katsumata Y, Ikari K, Buckner J, Robinson W, Seifert J, Feser M, Moss L, Norri J, Harigai M, Hsieh E, Holer M, Okamoto Y. Expansion of Circulating HLA-DR+ Peripheral Helper T Cells and CXCR5−CD38+ Mature Naïve B Cells in ACPA-positive Individuals At-risk for and with Classified RA [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/expansion-of-circulating-hla-dr-peripheral-helper-t-cells-and-cxcr5%e2%88%92cd38-mature-naive-b-cells-in-acpa-positive-individuals-at-risk-for-and-with-classified-ra/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/expansion-of-circulating-hla-dr-peripheral-helper-t-cells-and-cxcr5%e2%88%92cd38-mature-naive-b-cells-in-acpa-positive-individuals-at-risk-for-and-with-classified-ra/