Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Studies indicate that sex (male vs female) is predictive of outcomes with PsA treatments, such as TNF inhibitors (TNFi).1 Tofacitinib is an oral JAK inhibitor for the treatment of PsA. This post hoc analysis evaluated the impact of sex on tofacitinib efficacy and safety in PsA.
Methods: Data were pooled from two Phase 3, randomized, placebo-controlled studies of tofacitinib (OPAL Broaden [NCT01877668]; OPAL Beyond [NCT01882439]) in patients (pts) with active PsA and an inadequate response to ≥ 1 conventional synthetic DMARD (and were TNFi-naïve; OPAL Broaden) or ≥ 1 TNFi (OPAL Beyond). Analyses included pts randomized to tofacitinib 5 mg twice daily (BID), tofacitinib 10 mg BID, or placebo (switching to tofacitinib 5 or 10 mg BID at Month [M]3). Efficacy endpoints included ACR20/50 response rates; change from baseline (Δ) in Leeds Enthesitis Index (LEI), ΔDactylitis Severity Score (DSS), and ΔFunctional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F); and proportion of pts achieving a minimal clinically important difference (MCID) for HAQ-Disability Index (HAQ-DI) (≥ 0.35). Safety outcomes were assessed up to M6. Demographic and baseline disease characteristics were tested using one-way analysis of variance for continuous parameters and chi-squared test for categorical parameters. Analyses are based on observed cases and presented without p-value multiplicity adjustment.
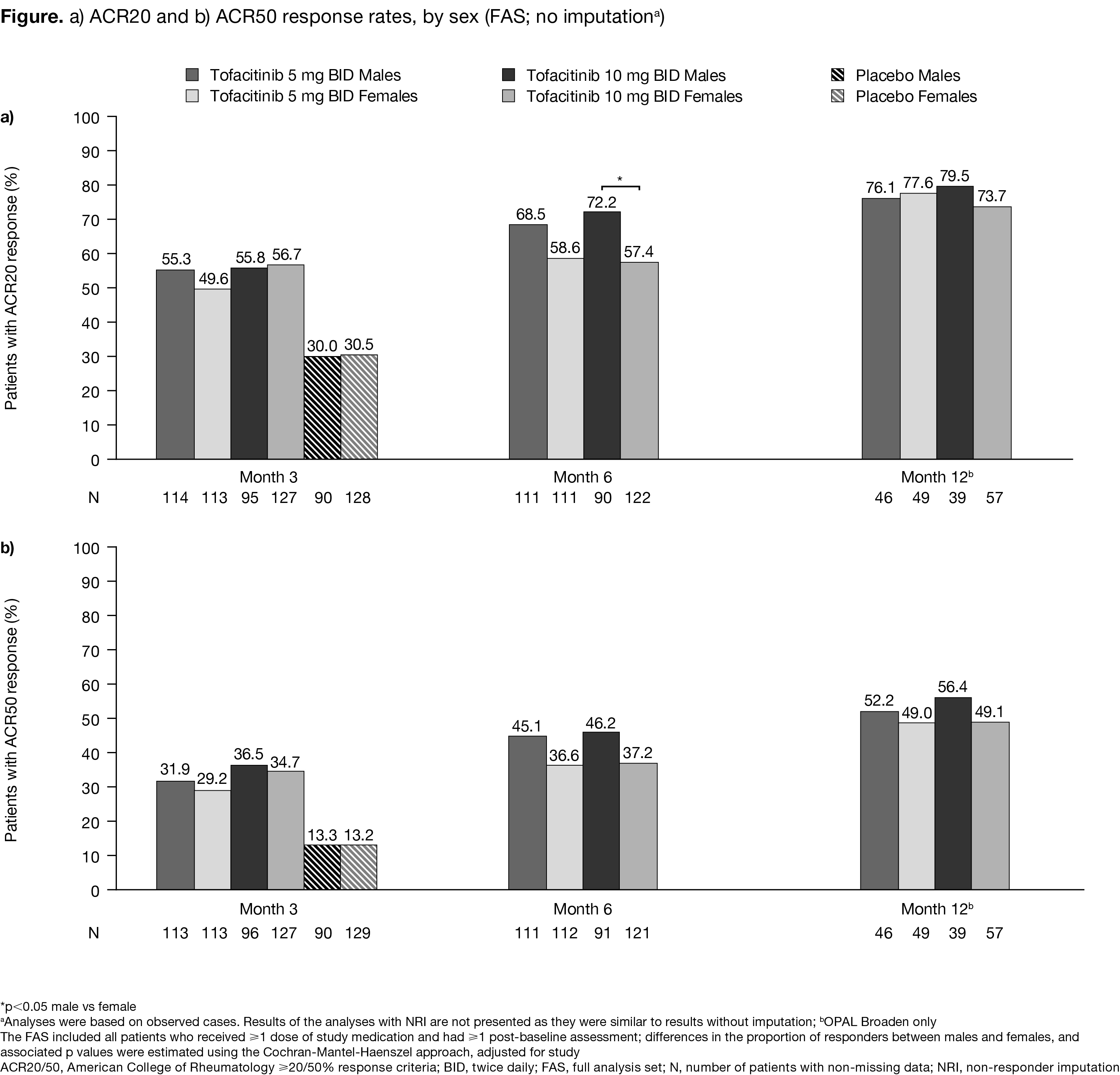
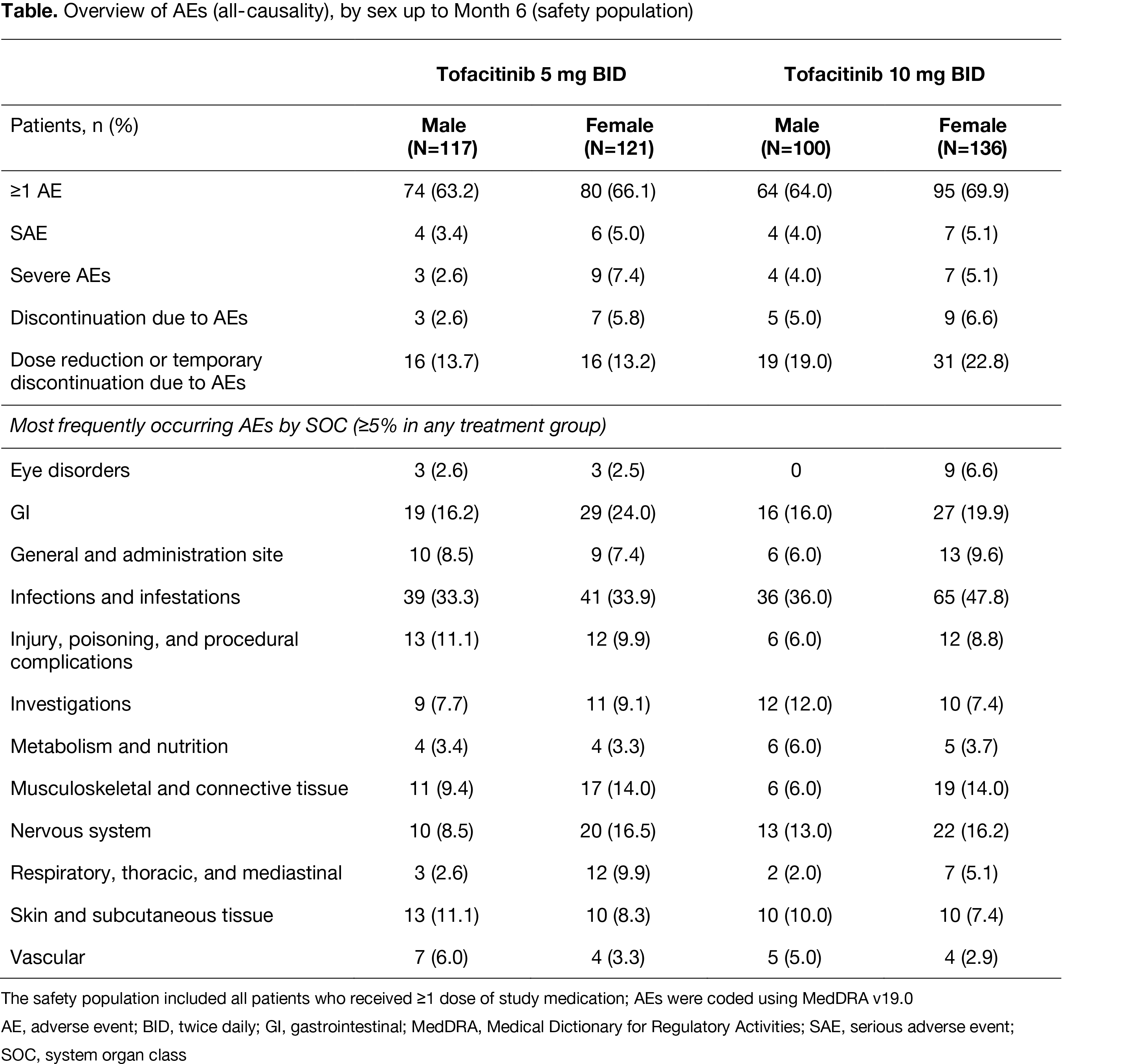
Results: Overall, 710 pts (317/393, male/female) were included (tofacitinib 5 mg BID, 117/121; tofacitinib 10 mg BID, 100/136; placebo, 100/136, respectively). Baseline characteristics were broadly similar between sexes; exceptions included higher Composite Psoriatic Disease Activity Index in females (tofacitinib 5 mg), higher FACIT-F total score (less fatigue) in males, higher HAQ-DI in females (tofacitinib 5 mg BID and placebo), and higher DSS in males (placebo). ACR20/50 response rates were similar between sexes at M3. At M6, ACR20 response rates were numerically higher for males (Figure a), with a significant difference seen for tofacitinib 10 mg BID (72.2% males vs 57.4% females; p=0.0163); no significant differences in ACR50 response rates were observed (Figure b). ACR20/50 response rates were similar between sexes by M12 (OPAL Broaden). In general, no significant differences between sexes were shown in ΔLEI, ΔDSS, ΔFACIT-F, or HAQ-DI MCID at M3, 6, and 12 in all groups. Up to M6, numerically more females vs males had adverse events (AEs), serious AEs, severe AEs, discontinuations due to AEs, gastrointestinal, nervous, respiratory, thoracic and mediastinal system disorders (system organ class) with tofacitinib 5 and 10 mg BID (Table). Rates of vascular disorders were numerically higher in males.
Conclusion: In general, no clinically meaningful differences between sexes were observed in the efficacy of tofacitinib 5 and 10 mg BID up to M12. Some numerical differences in safety outcomes with tofacitinib were seen between sexes. Results further characterize the efficacy and safety of tofacitinib in pts with PsA.
1. Eder L, Gladman DD. Expert Rev Clin Immunol 2014; 10: 763-770.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by Jennifer Arnold, CMC Connect and funded by Pfizer Inc.
To cite this abstract in AMA style:
Eder L, Gladman D, Zehra Aydin S, Ogdie A, Shi H, Landry P, Luna R. Evaluation of Sex Differences in the Efficacy and Safety of Tofacitinib in Patients with Active Psoriatic Arthritis: A Post Hoc Analysis of Two Phase 3 Randomized Controlled Trials [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/evaluation-of-sex-differences-in-the-efficacy-and-safety-of-tofacitinib-in-patients-with-active-psoriatic-arthritis-a-post-hoc-analysis-of-two-phase-3-randomized-controlled-trials/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-sex-differences-in-the-efficacy-and-safety-of-tofacitinib-in-patients-with-active-psoriatic-arthritis-a-post-hoc-analysis-of-two-phase-3-randomized-controlled-trials/