Session Information
Session Type: Abstract Session
Session Time: 10:00AM-10:50AM
Background/Purpose: Pneumococcal vaccination is recommended in patients with RA who are receiving conventional synthetic/biologic DMARDs.1 Upadacitinib (UPA) is an oral Janus kinase (JAK) inhibitor engineered to have a greater selectivity for JAK1 versus JAK2, JAK3, and tyrosine kinase 2, and is approved for the treatment of RA. The aim of this analysis was to assess the impact of long-term treatment with UPA + background MTX on immunologic responses to Prevnar 13® (Pneumococcal 13-valent Conjugate Vaccine [Diphtheria CRM197 Protein]; PCV13) in patients with RA enrolled in the ongoing Phase 2 open-label extension study BALANCE-EXTEND.
Methods: Patients from BALANCE-EXTEND receiving PCV13 vaccination were required to be on UPA 15 mg once daily (QD) or 30 mg QD and background MTX for ≥4 weeks prior to, and after, PCV13 vaccination; MTX was not interrupted prior to vaccination. Vaccination antibody titers were collected pre-vaccination (Week 0) and post-vaccination (Weeks 4 and 12). The primary variable was the proportion of patients with satisfactory humoral response to PCV13 (≥2‑fold increase in antibody concentration from pre-vaccination [Week 0] in ≥6/12 pneumococcal antigens [1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F]) at 4 weeks post-vaccination.
Results: Of 111 patients (UPA 15 mg, n=87; UPA 30 mg, n=24), 86% were female, most (98%) were white, and mean (standard deviation) age was 58.4 (12.0) years. Prior to vaccination, patients had a median (range) duration of RA of 9.3 (3.4–35.0) years and had been receiving UPA for a median (range) of 3.9 (3.0–4.9) years. All but 3 patients were taking concomitant MTX, and 44.1% were taking a CS (median daily dose, 5.0 mg). All 111 patients received PCV13, none discontinued UPA during the first 4 weeks, and blood samples were available from 83/23 and 79/22 patients in the UPA 15/30 mg groups at Weeks 4 and 12, respectively. At 4 weeks, satisfactory humoral response to PCV13 occurred in 67.5% (95% confidence interval [CI]: 57.4–77.5) and 56.5% (95% CI: 36.3–76.8) of patients receiving UPA 15 and 30 mg, respectively. At 12 weeks, satisfactory humoral response to PCV13 occurred in 64.6% (95% CI: 54.0–75.1) and 54.5% (95% CI: 33.7–75.4) of patients receiving UPA 15 and 30 mg, respectively (Figure 1). There was no clear difference in response between patients receiving and not receiving concomitant CS. Within 30 days post-vaccination, 2 adverse events (AEs) were considered as possibly related to UPA (1 case of diverticulitis, UPA 15 mg; 1 case of anemia, UPA 30 mg) and no serious AEs were reported (Table 1). Two patients experienced pyrexia and 1 subject each experienced vaccination-site pain and headache within 1 day post-vaccination (all in UPA 15 mg group).
Conclusion: Satisfactory humoral response to PCV13 at 4 weeks occurred in ~two-thirds of patients with RA receiving long-term treatment with UPA 15 mg QD + background MTX. This is broadly consistent with pneumococcal vaccine humoral responses observed in patients with RA treated with other JAK inhibitors, biologics, or placebo.2–4
- Singh JA, et al. Arthritis Care Res 2016;68:1–25.
- Winthrop KL, et al. Arthritis Res Ther 2019;21:102.
- Bingham CO, et al. Ann Rheum Dis 2015;74:818–22.
- Winthrop KL, et al. Ann Rheum Dis 2016;75:687–95.
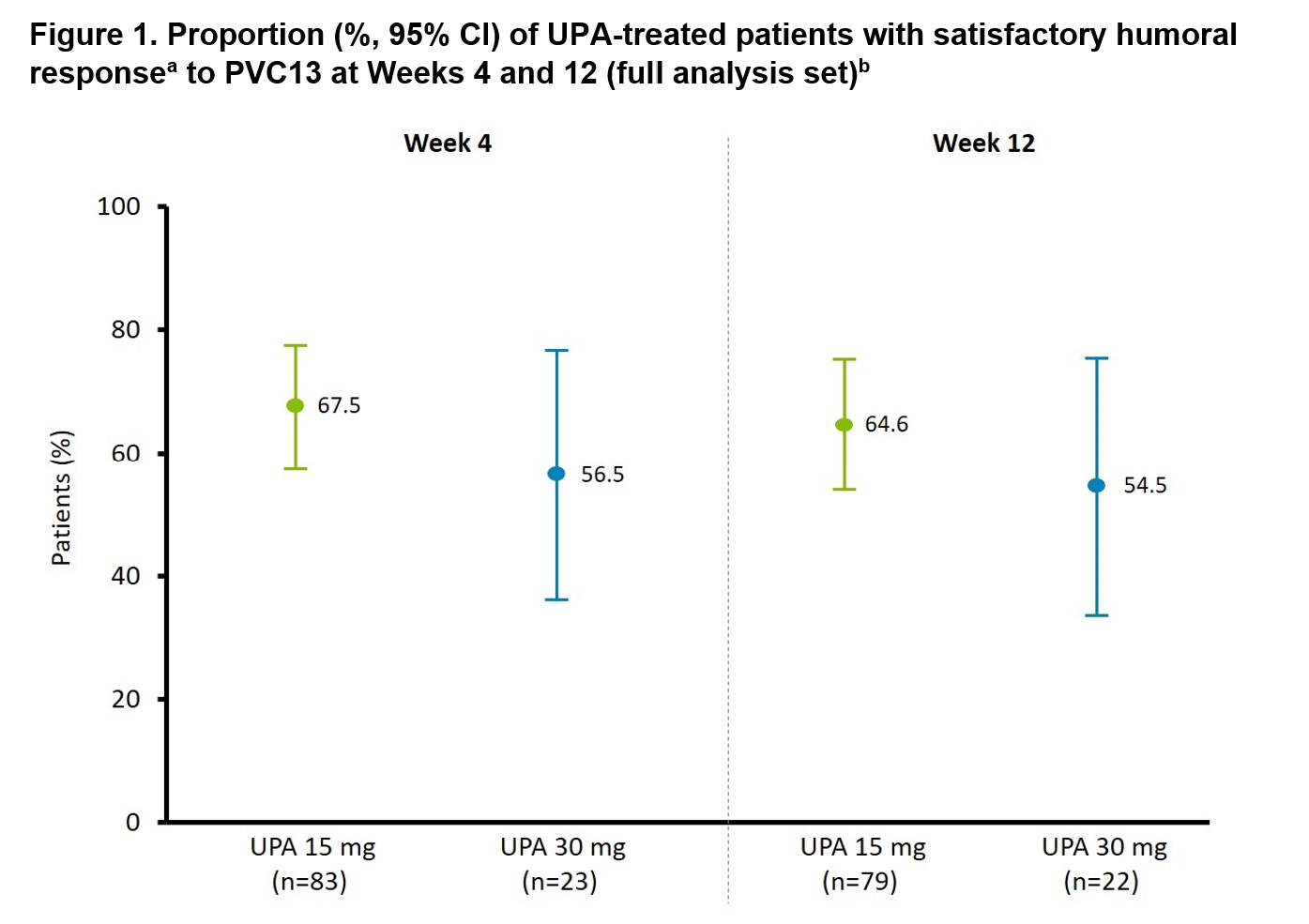 ᵃSatisfactory humoral response was defined as ≥2‑fold increase in antibody concentration from the vaccination baseline in ≥6 out of 12 pneumococcal antigens (1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F). ᵇNumber of patients based on availability of blood samples collected at Weeks 4 and 12. CI, confidence interval; PVC13, Pneumococcal 13-valent Conjugate Vaccine [Diphtheria CRM₁₉₇ Protein]; UPA, upadacitinib
ᵃSatisfactory humoral response was defined as ≥2‑fold increase in antibody concentration from the vaccination baseline in ≥6 out of 12 pneumococcal antigens (1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F). ᵇNumber of patients based on availability of blood samples collected at Weeks 4 and 12. CI, confidence interval; PVC13, Pneumococcal 13-valent Conjugate Vaccine [Diphtheria CRM₁₉₇ Protein]; UPA, upadacitinib
 ᵃAs assessed by the investigator. ᵇDiverticulitis. ᶜAnemia. AE, adverse event; PVC13, Pneumococcal 13-valent Conjugate Vaccine [Diphtheria CRM₁₉₇ Protein]; QD, once daily; UPA, upadacitinib.
ᵃAs assessed by the investigator. ᵇDiverticulitis. ᶜAnemia. AE, adverse event; PVC13, Pneumococcal 13-valent Conjugate Vaccine [Diphtheria CRM₁₉₇ Protein]; QD, once daily; UPA, upadacitinib.
To cite this abstract in AMA style:
Winthrop K, Vargas J, Drescher E, Garcia C, Friedman A, Enejosa J, Khan N, Li Y, Klaff J, Kivitz A. Evaluation of Response to Pneumococcal Vaccination in Patients with Rheumatoid Arthritis Receiving Upadacitinib: Results from a Phase 2 Open-Label Extension Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/evaluation-of-response-to-pneumococcal-vaccination-in-patients-with-rheumatoid-arthritis-receiving-upadacitinib-results-from-a-phase-2-open-label-extension-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-response-to-pneumococcal-vaccination-in-patients-with-rheumatoid-arthritis-receiving-upadacitinib-results-from-a-phase-2-open-label-extension-study/
