Session Information
Date: Tuesday, November 14, 2023
Title: (1945–1972) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Dermatomyositis (DM) is an immune-mediated inflammatory myopathy (IIM). Two subsets ofautoantibodies have been identified in patients with IIM: Myositis-specific antibodies (MSA) and myositis-associated antibodies (MAA). Whereas MSA are highly specific for IIMs and represent unique clinical phenotypes and prognosis, MAA are also found in other autoimmune diseases. The ProDERM study recently demonstrated the efficacy and safety of IVIG in DM patients (1) but the potential effect of autoantibodies on treatment response has not yet been determined. This post-hoc analysis of the randomized, placebo-controlled ProDERM study investigated autoantibody status at baseline and its relationship to treatment response to IVIG.
Methods: DM patients received 2 g/kg IVIg treatment (n=47) or placebo (n=48) every 4 weeks for 16 weeks. From week 16 onwards eligible patients (N=91) received IVIG for a further 24 weeks. The primary endpoint was a Total Improvement Score (TIS) of at least 20 (= at least minimal improvement) at week 16 and no confirmed deterioration up to week 16. Serum samples were taken at baseline and analysed for MSA (Jo-1, PL-7, PL-12, OJ, EJ, SRP, Mi-2, TIF-1, MDA5, NXP2, MJ, SUMO) and MAA (PM-SCL, Ku, U1RNP, U2RNP, U3RNP, Ro/SSA) by RNA and protein immunoprecipitations performed by the Oklahoma Medical Research Foundation. Patients were stratified according to their antibody (Ab) status as MSA-positive (including those also MAA+), MAA-positive only, or no Ab detected (Ab -ve) at baseline. Proportion of patients with minimal (TIS≥20), or moderate/major (≥ 40) response were evaluated for all patients (IVIG & placebo group) as well as separately at week 16 and 40.
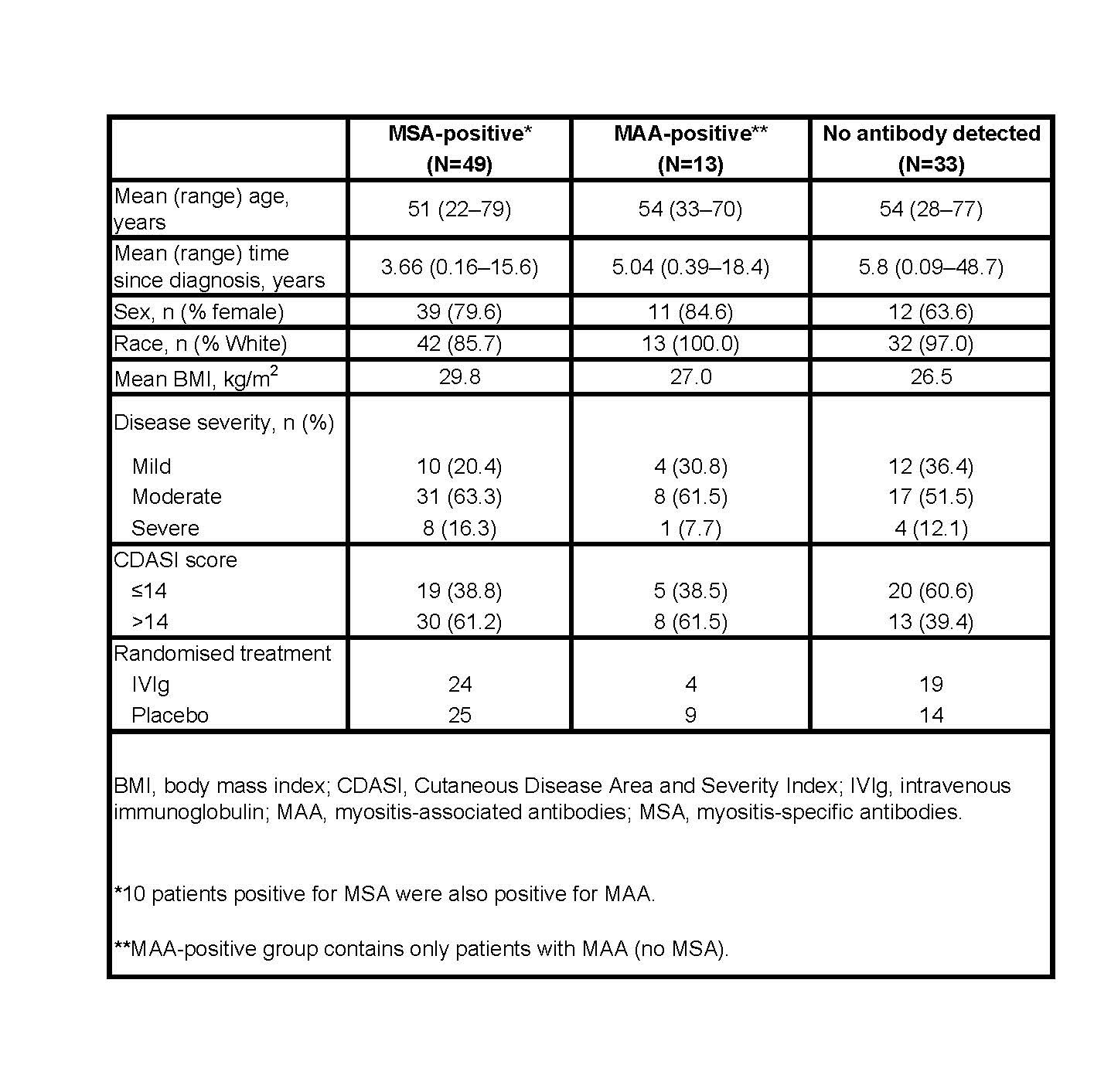
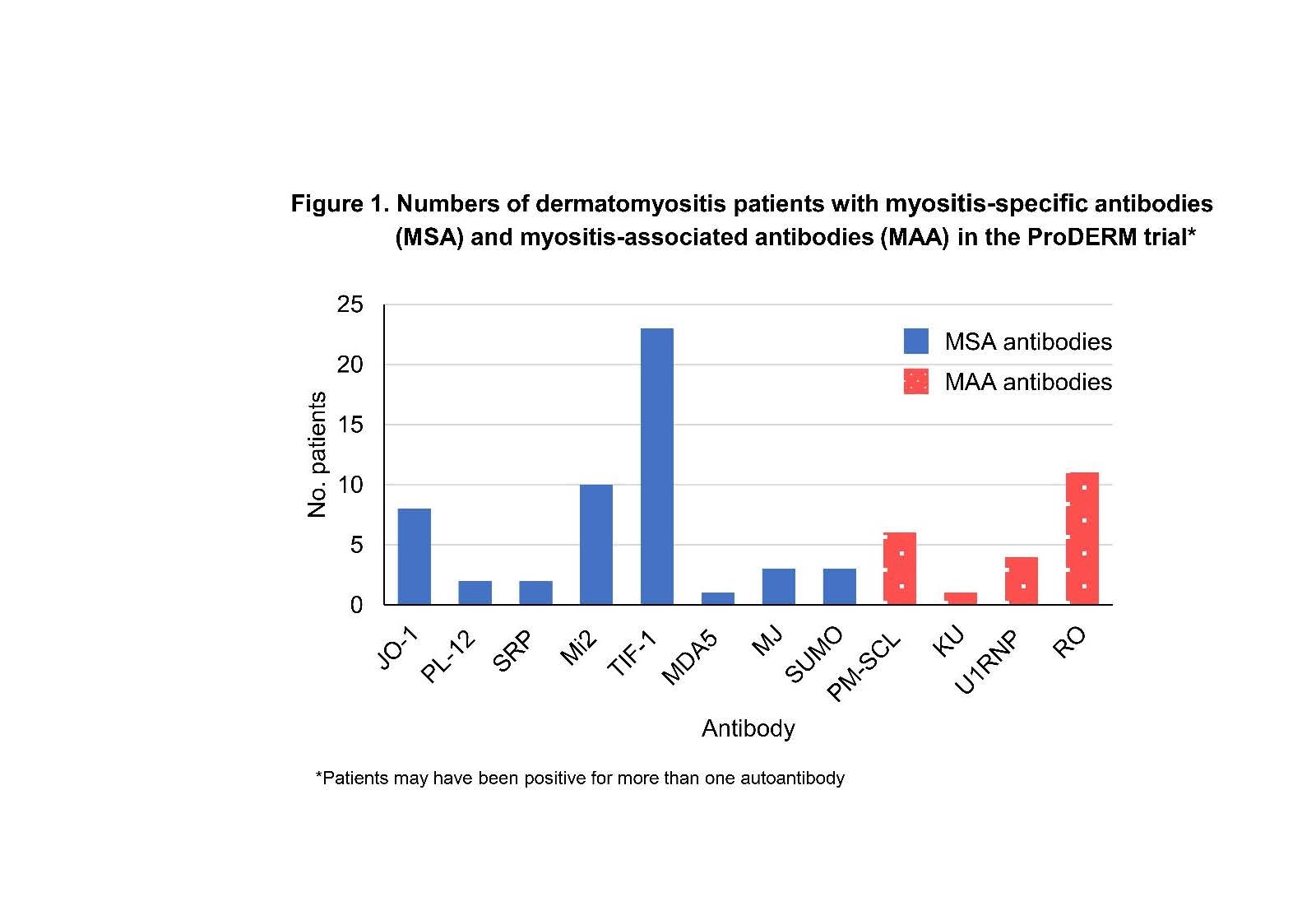
Results: At baseline, a total of 49 (52%) patients were MSA-positive, 13 (14%) were MAA-positive, and in 33(35%) no Ab were detected. Demographics of patients in each group are shown in Table 1. Ten MSA+ patients were also MAA+. Figure 1 shows numbers of patients with each specific Ab. In the MSA+group (both IVIG & placebo arms), 71% (35/49) of patients had minimal response at week 16, compared to 55% (18/33) in the Ab -ve group (p= 0.12) and 38% (5/13) in the MAA+ group (p = 0.03). Of the 24 MSA+ patients randomized to IVIG 83% showed TIS response at week 16 compared to 60% of MSA+ patients receiving placebo (p= 0.07). In the AB -ve group significantly more patients responded in the IVIG arm than in the placebo arm (p= 0.001). For all patients no significant difference between the 3 Ab groups was seen in percentage of patients with ‘moderate/major improvement’ at either Week 16 or Week 40. A subanalysis of individual Ab showed no significant difference in response rates of Ab+ vs. Ab -ve patients at week 16 or 40 for anti-synthetase Ab (N=10, Jo-1, PL-12) nor for anti-Mi2 Ab (N=10). However, significantly more anti-TIF-1 + patients (N=21) had minimal response compared to anti-TIF-1 -ve patients at week 16 and 40 (p=0.02 and p=0.03 respectively).
Conclusion: MSA positive patients showed a trend of higher frequency of minimal improvement but not of moderate to major improvement when compared to MAA+ or Ab negative patients. However, the response to IVIG was similar to placebo across the 3 antibody groups, suggesting that IVIG is effective irrespective of myositis autoantibody status.
To cite this abstract in AMA style:
Charles-Schoeman C, Schessl J, Aggarwal R, ProDERM investigators a. Evaluation of Myositis Autoantibodies as Predictors of Response to IVIG: Post-hoc Analysis of a Large Randomized, Double-Blind, Placebo-Controlled Phase III Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-myositis-autoantibodies-as-predictors-of-response-to-ivig-post-hoc-analysis-of-a-large-randomized-double-blind-placebo-controlled-phase-iii-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-myositis-autoantibodies-as-predictors-of-response-to-ivig-post-hoc-analysis-of-a-large-randomized-double-blind-placebo-controlled-phase-iii-trial/