Session Information
Date: Monday, November 13, 2023
Title: (1100–1123) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The multifaceted clinical presentation in crystal-induced arthropathies (CiA) poses challenges to imaging. Our goal was to formulate evidence-based recommendations on the use of imaging in the diagnosis and management of CiA.
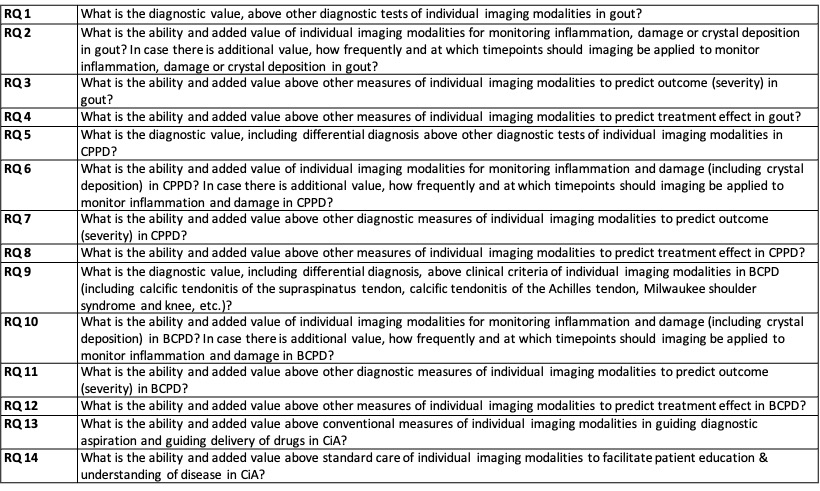
Methods: An international task force of 25 rheumatologists, radiologists, methodologists, health care professionals and patient research partners from 11 countries was formed according to the EULAR standard operating procedures. Fourteen key questions on the role of imaging in the most common forms of CiA were generated (Table 1). The CiA assessed included gout, calcium pyrophosphate dihydrate deposition and basic calcium phosphate deposition. Imaging modalities included conventional radiography, ultrasound, magnetic resonance imaging and computed tomography. Experts applied research evidence obtained from 4 systematic literature reviews using MEDLINE, EMBASE and CENTRAL after assessing for risk of bias. Task force members provided level of agreement (LoA) anonymously by using a numeric scale.
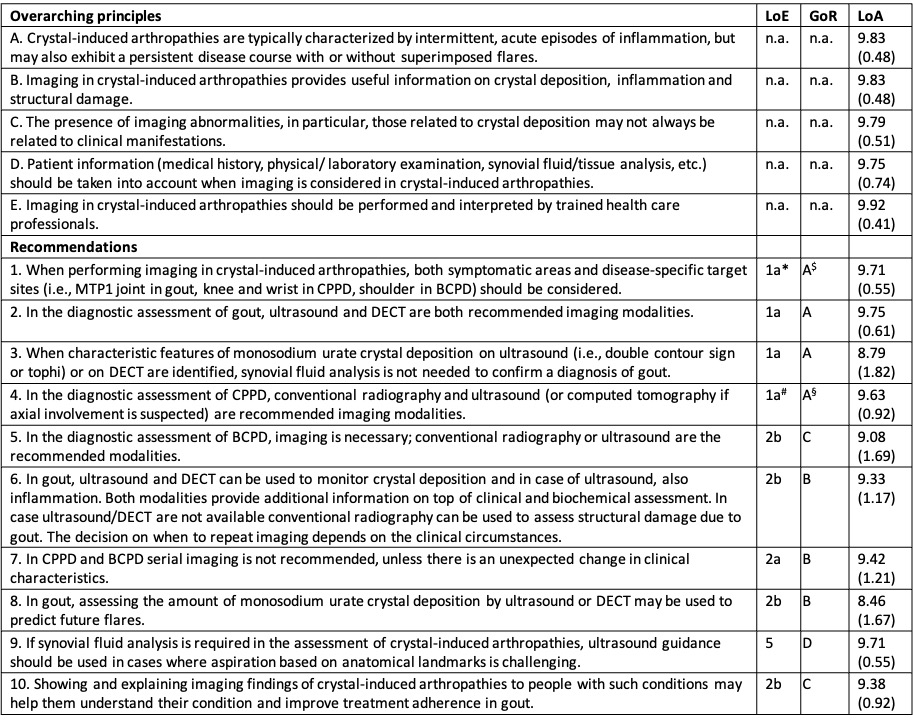
Results: Five overarching principles and 10 recommendations were produced on the role of imaging in making a diagnosis, monitoring inflammation, structural damage and crystal deposition, predicting severity and treatment effect, guiding intervention, and patient education in CiA (Table 2). Level of evidence and grade of recommendation was evaluated. Overall, the LoA for the recommendations was very high (8.5-9.9).
Conclusion: These are the first recommendations that encompass all common forms of CiA and guide the use of established imaging modalities in this disease group.
Numbers in column ‘LoA’ indicate the mean and SD (in parenthesis) of the LoA (range 0–10 with 0=‘completely disagree’ to 10=‘completely agree’),
BCPD: basic calcium phosphate deposition; CPPD: calcium pyrophosphate dihydrate deposition; DECT: dual-energy computed tomography; GoR: grades of recommendation; LoA: level of agreement; LoE: level of evidence; MTP1: first metatarsophalangeal; n.a., not applicable;
To cite this abstract in AMA style:
Mandl P, D'Agostino M, Navarro-Compán V, Gessl I, Sakellariou G, Abhishek A, Becce F, Dalbeth N, Ea H, Filippucci E, Hammer H, Iagnocco A, De Thurah A, Naredo E, Ottaviani S, Pascart T, Perez-Ruiz F, Pitsillidou I, Proft F, Rech J, Schmidt W, Sconfienza L, Terslev L, Wildner B, Zufferey P, Filippou G. EULAR Recommendations for the Use of Imaging in the Diagnosis and Management of Crystal-induced Arthropathies in Clinical Practice [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/eular-recommendations-for-the-use-of-imaging-in-the-diagnosis-and-management-of-crystal-induced-arthropathies-in-clinical-practice/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/eular-recommendations-for-the-use-of-imaging-in-the-diagnosis-and-management-of-crystal-induced-arthropathies-in-clinical-practice/