Session Information
Date: Tuesday, October 28, 2025
Title: Abstracts: Metabolic & Crystal Arthropathies – Basic & Clinical Science (2585–2590)
Session Type: Abstract Session
Session Time: 1:15PM-1:30PM
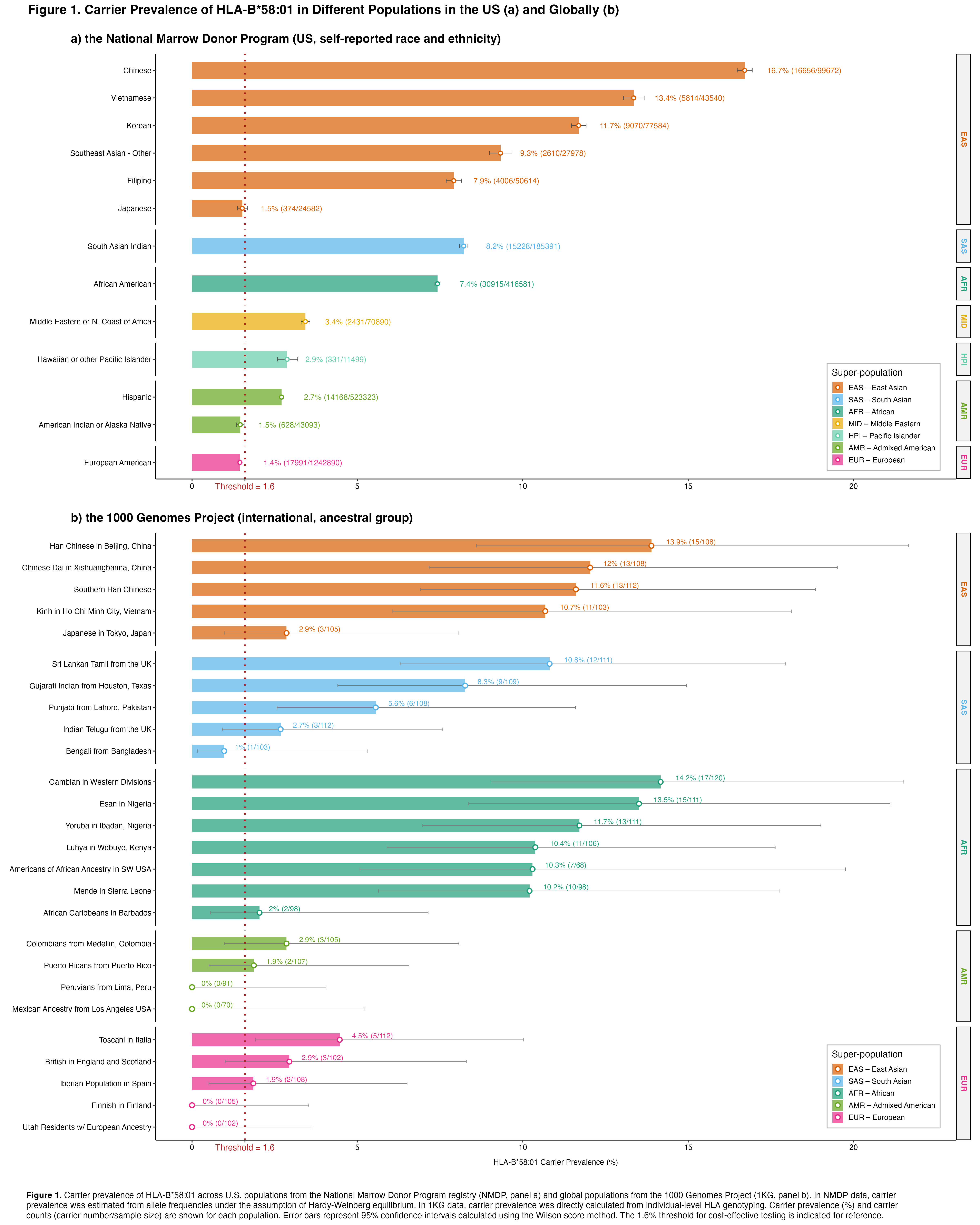
Background/Purpose: The HLA-B*58:01 is strongly associated with allopurinol-induced severe cutaneous adverse reactions. The 2020 American College of Rheumatology (ACR) Guideline for the Management of Gout conditionally recommends HLA-B*58:01 testing prior to initiating allopurinol in patients of certain Asian or African descent, while advising against routine testing in other ethnic groups. These recommendations are based on reported carrier prevalence and cost-effectiveness analyses. However, data remain limited for populations such as Native American, South Asian, and Middle Eastern groups. This study aimed to estimate the prevalence of HLA-B*58:01 carriers across diverse U.S. and global populations.
Methods: We performed a descriptive analysis of HLA-B*58:01 carrier prevalence across diverse populations using two data sources: (1) the National Marrow Donor Program (NMDP) , a large U.S. hematopoietic cell registry of approximately 2.9 million individuals. Allele frequencies, estimated via a modified expectation–maximization algorithm from mixed-resolution HLA typing data, were publicly available (Gragert et al., 2013). Carrier prevalence was inferred assuming Hardy-Weinberg equilibrium; and (2) the 1000 Genomes Project (1KG) , an international consortium that includes 2,693 individuals with publicly available four-digit HLA typing data. In the NMDP, population categories are based on self-reported race and ethnicity, whereas 1KG populations were sampled by self-identified ancestry and are widely used as genetic ancestry reference groups. We compared carrier prevalence to 1.6%, the threshold for cost-effective testing based on a prior modeling study cited in the ACR guideline (Jutkowitz et al., 2017).
Results: In the NMDP, estimated HLA-B*58:01 carrier prevalence exceeded the 1.6% threshold for cost-effective testing in individuals self-identifying as Chinese (16.7%, 95%CI 16.5%-16.9%), Vietnamese (13.4%, 95%CI 13.0%-13.7%), Korean (11.7%, 95%CI 11.5%-11.9%), other Southeast Asian (9.3%, 95%CI 9.0%-9.7%), Filipino (7.9%, 95%CI 7.7%-8.1%), South Asian Indian (8.2%, 95%CI 8.1%-8.3%), African American (7.4%, 95%CI 7.3%-7.5%), Middle Eastern or North African (3.4%, 95%CI 3.3%–3.6%), Hawaiian or other Pacific Islander (2.9%, 95%CI 2.6%-3.2%), and Hispanic (2.7%, 95%CI 2.7%-2.8%) (Figure 1a). In contrast, the estimated prevalence was below the 1.6% threshold for cost-effective testing among Japanese (1.5%, 95%CI 1.4%-1.7%), American Indian or Alaska Native (1.5%, 95%CI 1.4%-1.6%), and European American (1.4%, 95%CI 1.4%-1.5%). Carrier frequencies in ancestry-based populations from 1KG were largely consistent with NMDP estimates (Figure 1b).
Conclusion: Estimated HLA-B*58:01 carrier prevalence varies by race and ethnicity, with most groups exceeding the 1.6% threshold for cost-effective testing. Among those below the threshold, prevalence was only marginally lower. These findings raise the possibility that broader HLA-B*58:01 testing than recommended by the 2020 ACR guideline could be considered prior to allopurinol initiation.
To cite this abstract in AMA style:
Xu Q, Bathon J, Luo Y. Estimated Carrier Prevalence of HLA-B*58:01 Across Diverse Populations in the US and Globally [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/estimated-carrier-prevalence-of-hla-b5801-across-diverse-populations-in-the-us-and-globally/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/estimated-carrier-prevalence-of-hla-b5801-across-diverse-populations-in-the-us-and-globally/