Session Information
Date: Monday, November 13, 2023
Title: (0859–0885) Osteoarthritis & Joint Biology – Basic Science Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
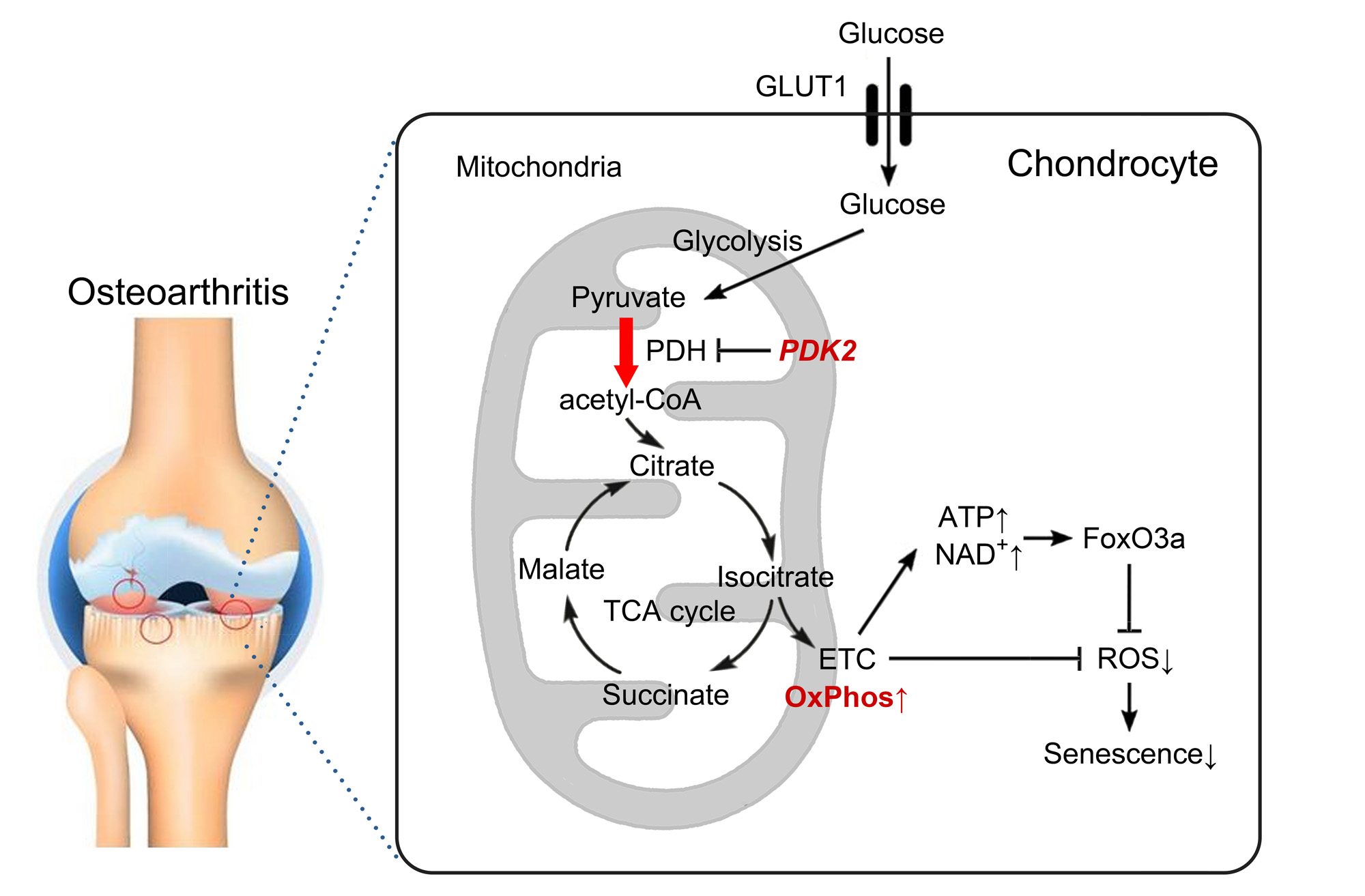
Background/Purpose: Although chondrocytes rely primarily on glycolysis to meet their cellular energy needs, they have the metabolic flexibility to shift toward oxidative phosphorylation (OxPhos) in the early stage of osteoarthritis (OA). As the disease progresses, however, this metabolic adaptation of OA cartilage reaches its limit and eventually fails, resulting in mitochondrial dysfunction and oxidative stress which are hallmarks of OA. The objective of this study was to determine whether the modulation of OxPhos through pyruvate dehydrogenase kinase (PDK)-2 affects metabolic flexibility of chondrocytes, and cartilage degeneration in surgical model of OA.
Methods: Primary chondrocytes were obtained from E15.5 C57BL/6J long bones of PDK2 KO mice and their WT littermates. Interleukin (IL)-1β (10 ng/mL) was applied to recapitulate the catabolic stress mimicking OA conditions. Surgical OA was induced in 12-week-old male WT (C57BL/6J) and PDK2 KO mice by destabilization of the medial meniscus (DMM). Metabolic shift by PDK2 deficiency was analyzed with XF96 Extracellular Flux Analyzer.
Results: Among PDK isoforms (PDK1-4), only PDK2 expression was increased by IL-1β at both RNA and protein levels in vitro, and in the articular cartilage of the DMM OA model in vivo. PDK2-deficient mice exhibited significant protection from DMM-induced cartilage destruction, which was accompanied by a decrease in oxidative stress and pain-related behaviors. PDK2 deficiency partially restored oxidative phosphorylation and decreased glycolysis in IL-1β-treated chondrocytes, which led to an increase in ATP concentration, and the NAD+/NADH ratio. PDK2 deficiency significantly increased the phosphorylation of pyruvate dehydrogenase (PDH), the inactive form, which was accompanied by a decrease in reactive oxygen species (ROS) and chondrocyte senescence, as well as the expression of MMP-13 and IL-6 induced by IL-1β treatment. At the signaling level, PDK2 deficiency enhanced FoxO3a and decreased p38 signaling, but did not affect AMPK, mTOR, and NF-kB pathways upon IL-1β stimulation. FoxO3a knockdown masked the reduction of ROS and senescence brought about by PDK2 deficiency, implying that PDK2 effects are FoxO3a-dependent.
Conclusion: Our study provides the proof-of-concept of PDK2-mediated metabolic reprogramming of chondrocytes towards OxPhos as a new therapeutic strategy for OA.
To cite this abstract in AMA style:
Han S, Han J, kim Y, Kim Y, Park D. Enhancing Oxidative Phosphorylation Through PDK2 Depletion Alleviates Cartilage Degradation in Surgically Induced Osteoarthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/enhancing-oxidative-phosphorylation-through-pdk2-depletion-alleviates-cartilage-degradation-in-surgically-induced-osteoarthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/enhancing-oxidative-phosphorylation-through-pdk2-depletion-alleviates-cartilage-degradation-in-surgically-induced-osteoarthritis/