Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Recently, it was demonstrated that temporarily halting mycophenolate mofetil (MMF) for 1-week post-COVID-19 vaccination in systemic lupus erythematosus (SLE) patients improved humoral response, without exacerbating the underlying disease. Thus, we assessed the MMF discontinuation after each dose of the recombinant vaccine for herpes zoster (RZV or Shingrix®) in patients with autoimmune rheumatic diseases (ARD). Objective: To evaluate the effect of MMF withdrawal after RZV on increasing the immune response in ARD patients with controlled underlying disease.
Methods: In this prospective randomized phase 4 study of 230 ARD patients with well-controlled disease, patients received two intramuscular doses of RZV 6 weeks apart at D0 (V1) and D42 (V2). Randomization allocated patients into two groups: MMF-hold, where MMF was suspended for 1 week after each dose of RZV; MMF-maintain, where therapy remained stable. Immunogenicity was evaluated at baseline (V1) and after 12 weeks (V3) and disease activity was assessed at V1, V2 and V3. Humoral immunogenicity was measured by anti-gE antibody serum concentrations using an in-house ELISA. A humoral response to RZV was defined as anti-gE antibody concentration ≥4-fold the lower limit of detection (0.02 mIU/mL) in initially seronegative subjects and as an antibody concentration ≥4-fold the prevaccination concentration in initially seropositive subjects ( >0.02 mIU/mL). Geometric mean titer (GMT) was calculated from logarithm-transformed concentrations. Samples were tested in duplicates (range: 0.02 to 40 mIU/mL). Disease flare was defined as >3 points increase in Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI 2K) for SLE and clinical judgment and/or increase in therapy for the other ARDs.
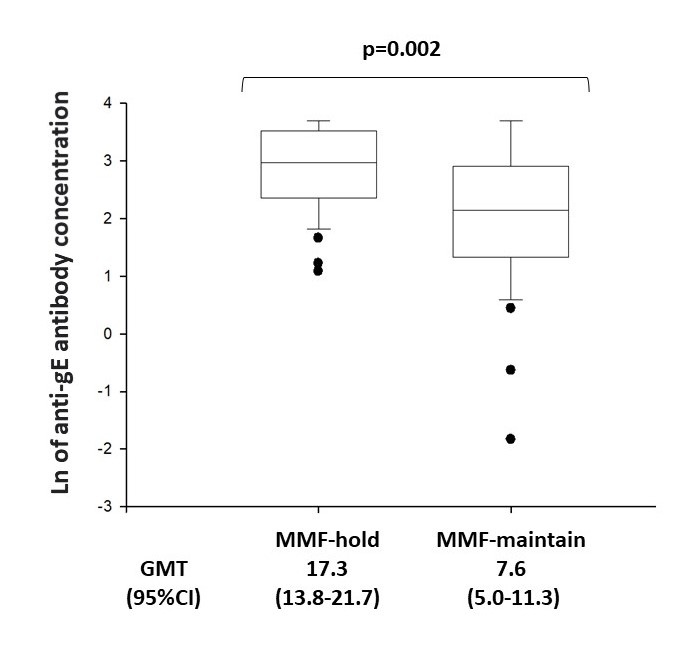
Results: In this interim analysis, among the 90 ARD (77 SLE, 9 systemic sclerosis and 4 myopathies) patients enrolled, 76 (39 in the MMF-hold group and 37 in the MMF-maintain group) had complete data through the first 12 weeks (V1 and V3). The two groups were similar in age (p=0.191), sex (p=0.082), ARD diagnosis (p >0.05) and concomitant therapies, including glucocorticoids (p=0.066) and biologic drugs use (p=0.929). At V3, the MMF-hold group exhibited significantly higher GMT [17.3 (95%CI 13.8-21.7) vs. 7.6 (95%CI 5.0-11.3) mIU/mL, p=0.002] (Figure 1), and a greater factor increase in GMT [51.7 (95%CI 31.3-85.3) vs. 20.1 (95%CI 12.7-31.6), p=0.03] compared to the MMF-maintain group. Flare rates were similar between the MMF-hold and MMF-maintain groups at V2 (12.8 vs. 2.7%, p=0.201) and V3 (20.5 vs. 5.4%, p=0.087). Patients in the MMF-hold group reported significantly higher incidences of local pain (p=0.013) (mean duration of 2.8 ± 1.5 days) after the first dose, however none of them had vaccine-related moderate or severe adverse events. No herpes zoster cases were reported up to week 12 in both groups.
Conclusion: Our study demonstrates that a short-term MMF discontinuation of 1 week following each dose of the RZV vaccine substantially enhances the humoral response, with no deterioration of disease activity. This innovative approach shows promise in boosting vaccine immunogenicity in ARD patients. (ClinicalTrials NCT05879419)
MMF = mycophenolate mofetil.
To cite this abstract in AMA style:
Pasoto S, Antonelli I, Medeiros-Ribeiro A, Aikawa N, Kupa L, Borba E, Seguro L, Figueiredo Neves Yuki E, ASSAD A, Saad C, Shimabuco A, Negrini A, Medeiros J, Ribeiro T, Shinjo S, Sampaio-Barros P, Andrade D, Souza F, Miossi R, Silva C, Bonfa E. Enhanced Immunogenicity of the Recombinant Herpes Zoster Vaccine After One-Week of Mycophenolate Mofetil Discontinuation in Patients with Autoimmune Rheumatic Diseases: Interim Results from a Prospective Randomized Phase 4 Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/enhanced-immunogenicity-of-the-recombinant-herpes-zoster-vaccine-after-one-week-of-mycophenolate-mofetil-discontinuation-in-patients-with-autoimmune-rheumatic-diseases-interim-results-from-a-prospect/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/enhanced-immunogenicity-of-the-recombinant-herpes-zoster-vaccine-after-one-week-of-mycophenolate-mofetil-discontinuation-in-patients-with-autoimmune-rheumatic-diseases-interim-results-from-a-prospect/