Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by synovial hyperplasia, consisting of type A macrophage-like synoviocytes and type B fibroblast-like synoviocytes (FLS). We have previously demonstrated that bone marrow (BM) CD34+ cells from RA patients have abnormal capacities to respond to tumor necrosis factor-α (TNF-α) and to differentiate into FLS, playing an important role in the pathogenesis of RA. However, the mechanism of the abnormal responses to TNF-α of BM CD34+ cells has been unclear. Of note, transforming growth factor β activated kinase 1 (TAK1) has been shown to be activated in the inflammatory signal transduction pathways in response to IL-1β, TNF-α or toll-like receptor stimulation. Moreover, it has been disclosed that intraarticular administration of siRNA for TAK1 inhibited the development of collagen-induced arthritis in mice. It is thus possible that aberrant expression of TAK1 in BM CD34+ cells might lead to their abnormal responses to TNF-α resulting in enhanced differentiation into FLS. The current study therefore examined the mRNA expression of TAK1 in BM CD34+ cells from RA patients.
Methods: BM samples were obtained from 47 patients with RA (6 males and 41 females: mean age 58.8 years) and 27 patients with OA (2 males and 25 females: mean age 70.4 years), who gave informed consent, during joint operations via aspiration from iliac crest. CD34+ cells were purified from the BM mononuclear cells by positive selection with magnetic beads. The expression of mRNA for TAK1 was examined by quantitative reverse transcription PCR. The results are shown as the ratio of the copy numbers to those of β-actin mRNA.
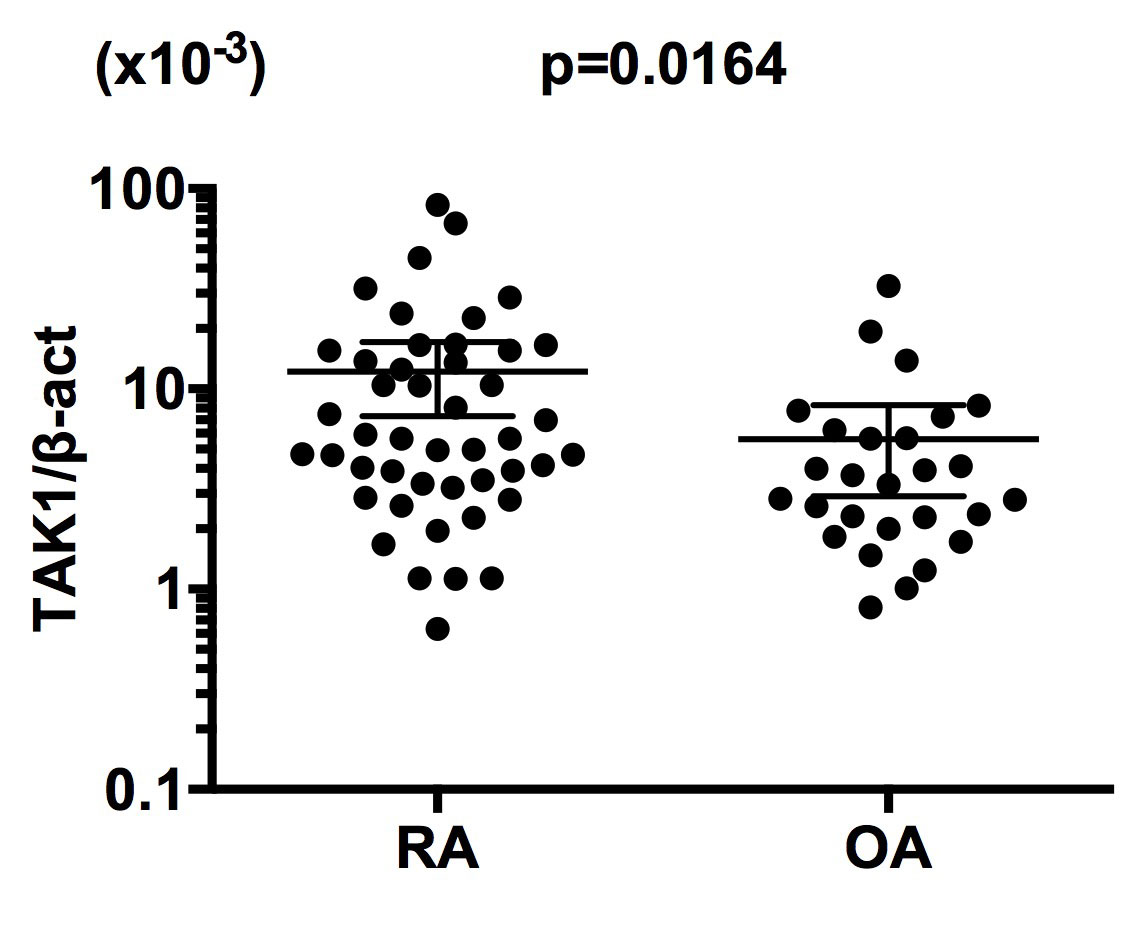
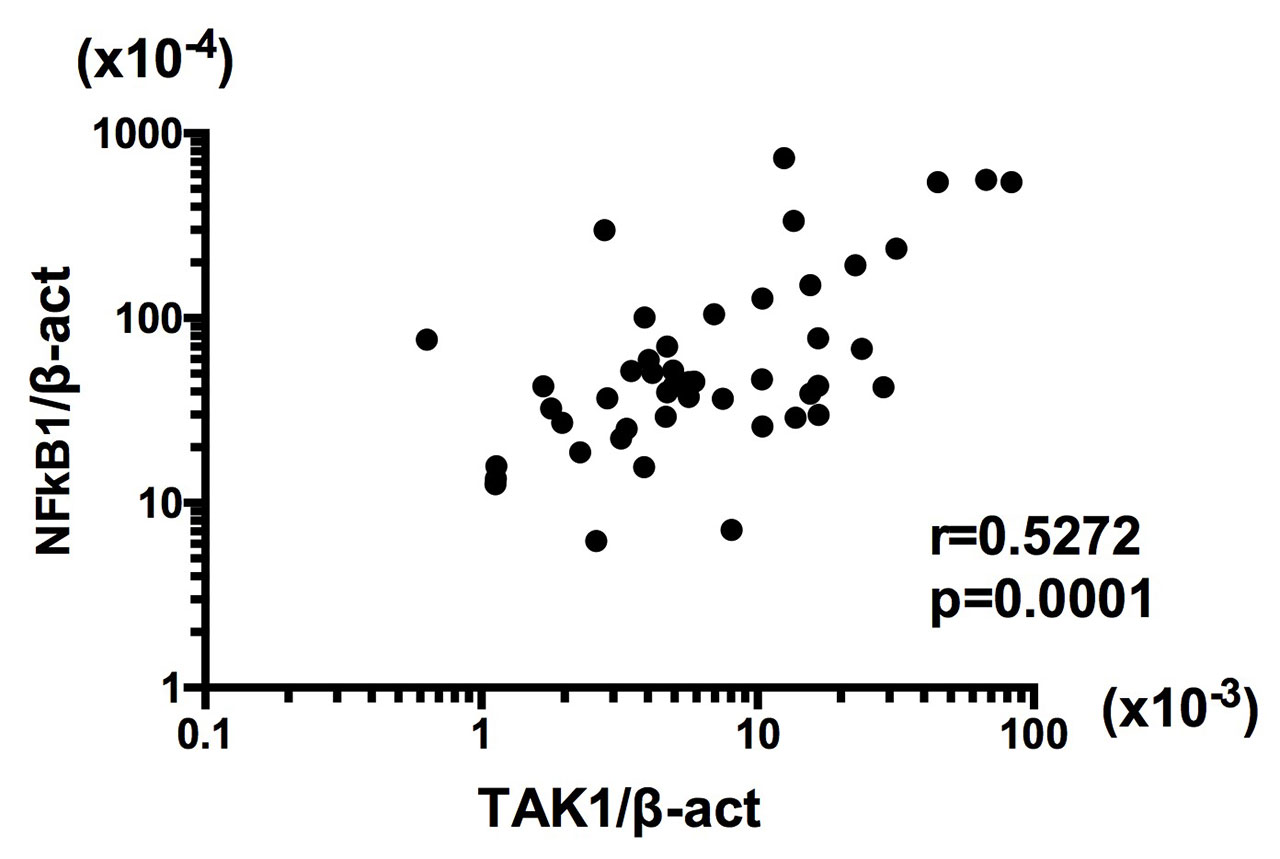
Results: The expression of mRNA for TAK1 was significantly higher in RA BM CD34+ cells than OA BM CD34+ cells (Figure 1). The mRNA expression levels of TAK1 were not correlated with serum C-reactive protein (r=0.08316, p=0.5784) or with treatment regimens with or without methotrexate or glucocorticoids. TAK1 mRNA expression was significantly correlated with NFκB1 mRNA expression in RA BM CD34+ cells (Figure 2). Finally, TNF-α enhanced NFκB1 mRNA expression, but not TAK1 mRNA expression, in BM CD34+ cells from normal individuals.
Conclusion: These results indicate that the enhanced expression of TAK1 mRNA in BM CD34+ cells plays a pivotal role in the pathogenesis of RA through upregulation of differentiation of FLS. Moreover, the data have also disclosed that the upregulation of TAK1 mRNA expression might lead to the increased expression of NFκB1 mRNA in BM CD34+ cells, but not vice versa, consistently with the position of TAK1 upstream of mitogen-activated protein kinases and the IkB kinase complex in signaling cascades. It is thus confirmed that TAK1 might be a good therapeutic target in RA.
To cite this abstract in AMA style:
Hirohata S, Nagai T, Tomita T, Yoshikawa H. Enhanced Expression of mRNA for TAK1 in CD34+ Cells of the Bone Marrow in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/enhanced-expression-of-mrna-for-tak1-in-cd34-cells-of-the-bone-marrow-in-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/enhanced-expression-of-mrna-for-tak1-in-cd34-cells-of-the-bone-marrow-in-rheumatoid-arthritis/