Session Information
Date: Sunday, October 26, 2025
Title: (0671–0710) Systemic Sclerosis & Related Disorders – Clinical Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Hand disability is a major feature of systemic sclerosis (SSc), driven by skin thickening and joint contractures, with significant impact on quality of life. Its assessment remains challenging and calls for more sensitive tools than traditional clinimetric scores. The Hand Test System (HTS) engineered glove has previously provided detailed data on hand dexterity in rheumatoid arthritis [1] through three quantitative parameters: touch duration (TD), inter-tapping interval (ITI), and movement rate (MR). The primary aim was to evaluate the applicability of the HTS glove in assessing hand dexterity in SSc patients by comparing its parameters with healthy controls (HC). Secondary endpoints included the correlations between HTS parameters with patient-reported outcomes (PROMs), nailfold videocapillaroscopy (NVC) findings, and dermal thickness measured by high frequency skin ultrasonography (HFSU) at the sites assessed by the modified Rodnan skin score (mRSS).
Methods: Twenty-five patients with systemic sclerosis (SSc) and 25 healthy controls were enrolled (Table 1). All participants underwent assessment using the HTS glove, grip strength measurement, and the following PROMs: the Health Assessment Questionnaire for Systemic Sclerosis (SHAQ), Hand Mobility in Scleroderma (HAMIS) test, and Duruöz Hand Index (DHI). SSc patients met the 2013 ACR/EULAR criteria and underwent standard clinical evaluation, nailfold videocapillaroscopy (NVC)[2] and HFSU (22 MHz), used to measure dermal thickness, with a focused analysis on the middle fingers, dorsum of hands, and total dermal thickness.
Results: In SSc patients, HTS parameters significantly correlated with PROMs, worsening in parallel with poorer PROMs (Table 2). Notably, both mean TD and ITI were directly correlated with the mRSS, whereas MR, as expected, was inversely correlated (Table 2). Grip strength showed a significant correlation only with mean ITI (r = -0.43, p = 0.03). As expected, significant differences in HTS parameters were observed between SSc patients and HC (Table 2). SSc patients performed finger movements more slowly and less efficiently than HC, as shown by prolonged TD and ITI, and reduced MR.When analyzing subgroups of SSc patients based on clinical features, no significant differences were detected in HTS parameters according to the different organ involvement, autoantibody profiles or NVC findings (Tables 2-3).However, significant correlations were, instead, observed between the mean TD directly correlating with the increase of the HFSU-measured dermal thickness of the dorsum of the hands and with the total dermal thickness (all p-values < 0.05, Table 2).
Conclusion: The HTS glove can provide detailed and quantitative data on hand dexterity in SSc patients. Significant correlations with PROMs, mRSS, hand strength and with HFSU-measured dermal thickness might suggest a significant relationship between disease-related skin thickness and hand functionality. Further studies with larger cohorts are in progression to explore the HTS glove’s utility in monitoring disease outcomes and therapeutic response in SSc. References[1] Patanè M et al. Joint Bone Spine. 2022 [2] Smith V et al. Aut Rev 2020
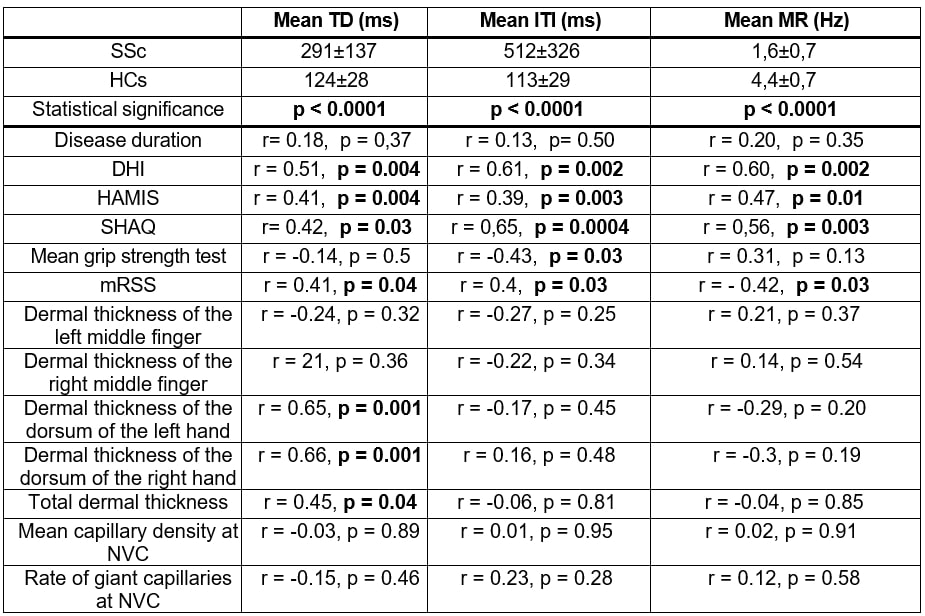 Table 1. Clinical and demographic features of the included patients
Table 1. Clinical and demographic features of the included patients
Legend. ACA; anti-centromere autoantibodies; dcSSc; diffuse cutaneus SSc, DMARDs; disease modifying anti-rheumatic drugs, lcSSc; limited cutaneous SSc, mRSS; modified Rodnan skin score, Scl-70; anti-topoisomerase I antibodies. * Patients were classified as with dcSSc if the involvement of the skin included also arms, trunk, and thighs.
Vasodilating agents: Aminaftone was taken by 17/25 (68%), calcium channel blockers by 6/25 (24%), endothelin receptor antagonists by 12/25 (48%), PDE5-inhibitors by 4/25 and riociguat by 1/25 (4%).
DMARDs: Mycophenolate mofetil was taken by 7/25 (28%), methotrexate by 5/25 (20%), rituximab by 2/25 (8%), hydroxychloroquine by 1/25 (4%), cyclophosphamide by 4/25 (16%), and low-dose (< or = 5 mg daily) prednisone by 6/25 (24%).
Pulmonary involvement: Defined based on the presence of ILD on high-resolution computed tomography (HRCT).
Gastroesophageal involvement: Defined based on manometric evidence of esophageal dysmotility.
Kidney involvement: Defined based on increased renal resistive index and/or elevated serum creatinine levels.
Significant p-values are reported in bold
.jpg) Table 2. Differences of the Hand Test System (HTS) engineered glove parameters between patients with systemic sclerosis (SSc) and healthy controls (HCs) Correlations between HTS glove parameters with clinimetric, nailfold videocapillaroscopy (NVC) and high frequency skin ultrasound (HFSU) parameters in SSc patients are also reported.
Table 2. Differences of the Hand Test System (HTS) engineered glove parameters between patients with systemic sclerosis (SSc) and healthy controls (HCs) Correlations between HTS glove parameters with clinimetric, nailfold videocapillaroscopy (NVC) and high frequency skin ultrasound (HFSU) parameters in SSc patients are also reported.
Legend. DHI; Duruöz Hand Index test, HAMIS; Hand Mobility in Scleroderma test, ILD; interstitial lung disease; ITI; inter-tapping interval, MR; movement rate, TD; touch duration, SHAQ; Health Assessment Questionnaire for Systemic Sclerosis.
Significant p-values are reported in bold
.jpg) Table 3. Subgroup analyses of glove parameters according to the clinical features and autoantibody profile
Table 3. Subgroup analyses of glove parameters according to the clinical features and autoantibody profile
Legend. See Table 1 and 2
To cite this abstract in AMA style:
Sulli A, Hysa E, Clini P, Gotelli E, Vojinovic T, Pizzorni C, Jaffal A, Paolino S, Campitiello R, Smith V, Cutolo M. Engineered glove for the objective assessment of hand dexterity in patients with systemic sclerosis: correlations with clinical features, nailfold videocapillaroscopy, and high frequency skin ultrasonography [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/engineered-glove-for-the-objective-assessment-of-hand-dexterity-in-patients-with-systemic-sclerosis-correlations-with-clinical-features-nailfold-videocapillaroscopy-and-high-frequency-skin-ultrason/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/engineered-glove-for-the-objective-assessment-of-hand-dexterity-in-patients-with-systemic-sclerosis-correlations-with-clinical-features-nailfold-videocapillaroscopy-and-high-frequency-skin-ultrason/
