Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
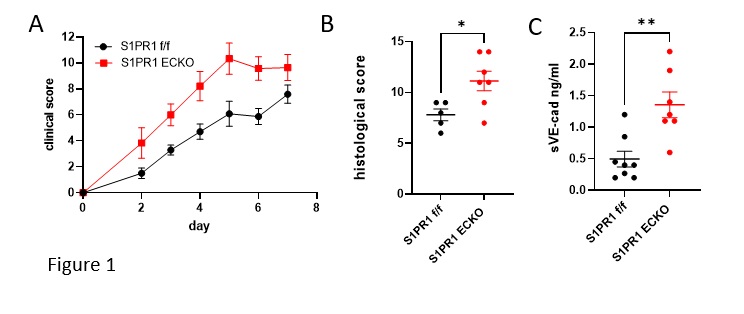
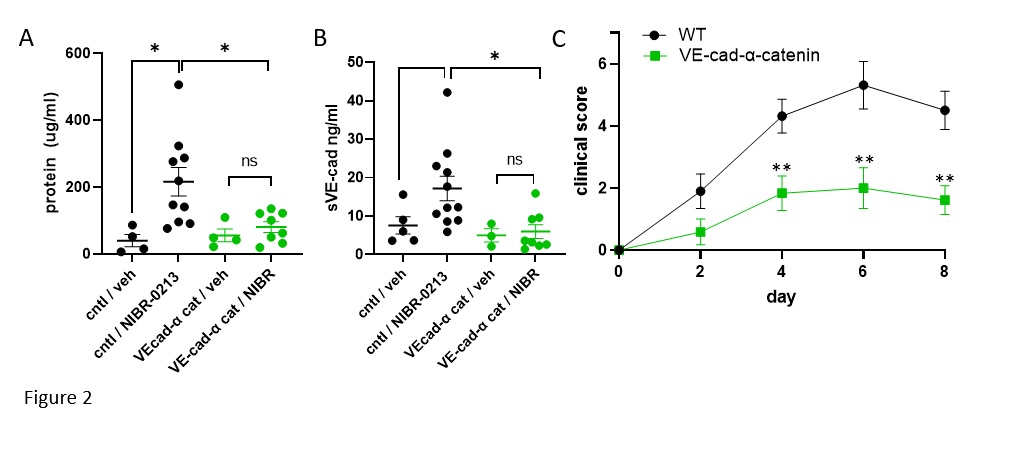
Background/Purpose: In rheumatoid arthritis, inflammatory mediators extravasate from blood into joints via gaps between endothelial cells (EC), but the contribution of ECs to inflammatory arthritis is not known. Sphingosine 1-phosphate receptor-1 (S1PR1), widely expressed on ECs, plays critical roles in EC barrier function and vascular health. Mice with an inducible EC-specific KO of S1PR1 (S1PR1 ECKO) had increased injury in response to SIA. Blockade of EC S1PR1 induced metalloproteinase-dependent cleavage of VE-cadherin in vitro. These data suggested that vascular leakage associated with S1PR1 inhibition, potentially a consequence of shedding of VE-cadherin, contributed to inflammatory injury. Therefore, we studied mice with highly stabilized EC junctions (VE-cad-α-cat mice) to test whether they resisted vascular permeability in the face of S1PR1 blockade and whether they would resist SIA. We also asked whether patients with active RA have dysregulated S1P/S1PR1 axis favoring vascular leakage.
Methods:
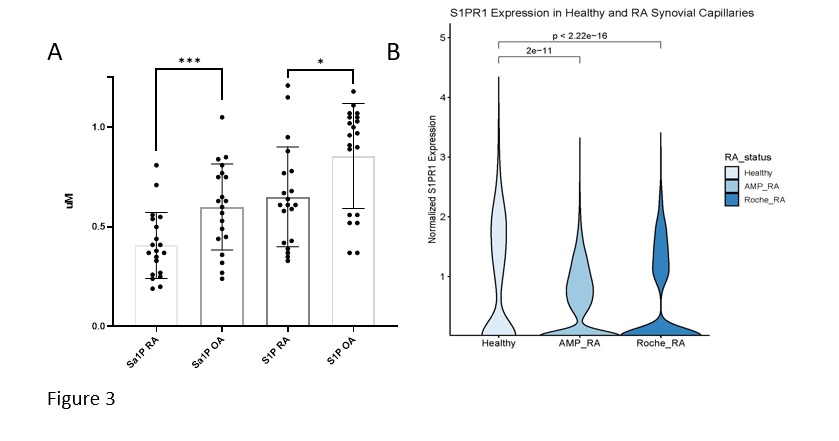
VE-cad-α-cat mice were treated with the S1PR1 antagonist NIBR-0213 (30 mg/kg IM) for 3 hours. We measured vascular leak and sVE-cad in bronchoalveolar fluids (BAL) with BCA assay and ELISA, respectively.VE-cad-α-cat, S1PR1 ECKO, and control mice were subjected to SIA (75 ul on days 0 and 2) and clinical scores were measured in a blinded fashion. For human studies, sera from 20 patients with RA and 20 age and sex matched patients with OA were analyzed for S1P and Sa1P by mass spectroscopy. Synovial EC S1PR1 was analyzed by single cell RNA seq in 3 data sets: (1) RA subjects in Accelerating Medicines Partnership Rheumatoid Arthritis / Systemic Lupus Erythematosus (AMP RA/SLE) Network, https://www.biorxiv.org/content/10.1101/2022.02.25.481990v1, (2) RA subjects from the The Roche Network for RA, and (3) healthy controls from Faust et al (https://doi.org/10.1101/2023.05.16.540975). Data sets were integrated and clusters of endothelial subtypes were identified using the top 10 marker genes for each cluster. Capillaries were subsetted for downstream visualization of S1PR1 expression. P-values were calculated using Wilcoxon rank-sum tests across RA statuses.
Results: S1PR1 ECKO mice had more severe SIA than littermate controls (Fig 1 A,B) along with increased extravascular sVE-cadherin in synovial tissues (Fig 1C). S1PR1 blockade induced less vascular leakage and extravascular VE-cadherin in the BAL in VE-cad-α cat mice compared to controls (Fig 2 A,B).VE-cad-α-cat mice were significantly protected from SIA (Fig 2C). Patients with active rheumatoid arthritis have decreased circulating S1P and Sa1P (Fig 3A), and their synovial microvascular ECs showed decreased expression of S1PR1 (Fig 3B) suggesting dysregulated S1P/S1PR1 axis in favor of vascular permeability and vulnerability.
Conclusion: We present a model in which EC S1PR1 signaling restrains the shedding of VE-cadherin to maintain homeostatic vascular barrier function and curb inflammation in experimental arthritis. Our hypothesis that vascular permeability is a clinical target in RA is supported by evidence that patients have dysregulated S1P/ S1PR1. We identify the microvascular barrier as a potential therapeutic target in inflammatory arthritis.
To cite this abstract in AMA style:
Burg N, Tran M, Wei K, Blobel C, Salmon J. Endothelial Cell Sphingosine 1-Phophate Receptor 1 Restrains VE-cadherin Cleavage and Attenuates Experimental Inflammatory Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/endothelial-cell-sphingosine-1-phophate-receptor-1-restrains-ve-cadherin-cleavage-and-attenuates-experimental-inflammatory-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/endothelial-cell-sphingosine-1-phophate-receptor-1-restrains-ve-cadherin-cleavage-and-attenuates-experimental-inflammatory-arthritis/