Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Tanezumab is a monoclonal antibody directed against nerve growth factor that is under study to treat moderate to severe chronic pain associated with osteoarthritis (OA) in adults for whom other treatments are ineffective or not appropriate. Phase 3 clinical trials have demonstrated the efficacy of subcutaneous (SC) tanezumab vs placebo for pain and function outcomes over various timepoints. Largely similar change from baseline was demonstrated in an oral NSAID-controlled study.1-3 The efficacy of some other OA therapies can be dampened in patients with depression, anxiety, or insomnia.4-6 A post hoc analysis explored efficacy of SC tanezumab after 16 weeks of treatment, as compared to oral NSAID, in patients with OA and a history of depression, anxiety, or insomnia at baseline.
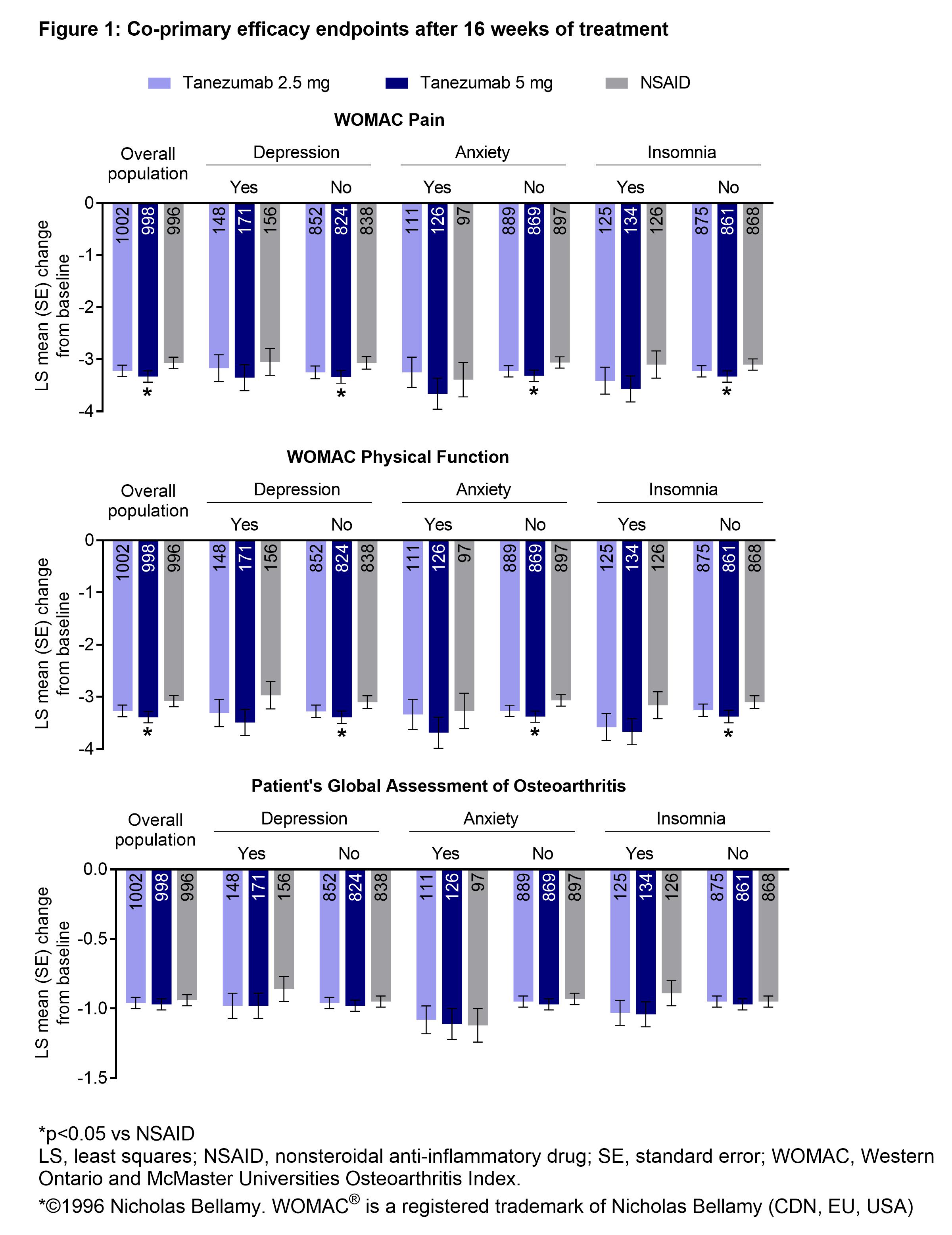
Methods: A randomized, double-blind, double-dummy, active-controlled phase 3 study (NCT02528188) of tanezumab SC 2.5 or 5 mg every 8 weeks vs twice daily oral NSAID in patients (aged ≥18 y) with radiographically confirmed moderate to severe hip or knee OA (KL grade ≥2).3 Co-primary efficacy endpoints were change from randomization to Week 16 in WOMAC Pain and Physical Function subscale scores (both ≥5/10 at baseline; higher scores indicate increasing pain/disability) and PGA-OA (≥3/5 at baseline; higher scores indicate poorer condition). Patients had a history of inadequate pain relief with acetaminophen; inadequate pain relief with/intolerance/contraindication to tramadol or opioids, or unwillingness to take opioids. Patients were on a stable dose of NSAID for ≥30 days before screening. Data are presented as least squares (LS) mean change from baseline to Week 16 for the whole population and subgroups of patients with/without a history of depression, anxiety, or insomnia at baseline. Statistical analysis by ANOVA (P not adjusted for multiplicity). This exploratory analysis was not prespecified or included in any sample size calculations; comparisons between treatment arms or patient subgroups should be interpreted with caution.
Results: Overall, 2996 patients were randomized and received at least one dose of study treatment (SC tanezumab 2.5 mg: n=1002; 5 mg: n=998; oral NSAID: n=996). In patients with or without a history of anxiety, depression, or insomnia, all treatments were associated with notable and largely similar magnitude improvements in WOMAC Pain and Physical Function and PGA-OA at Week 16 (Figure). Across treatment groups, differences in LS mean change from baseline in patients with and without a history of depression, anxiety, or insomnia ranged between 0–0.34 for WOMAC Pain and Physical Function and 0–0.19 for PGA-OA.
Conclusion: Patients with a history of depression, anxiety, or insomnia did not appear to experience dampened improvements in pain or function with tanezumab or NSAID vs those without.
Funded by Pfizer and Eli Lilly.
References:
1. Schnitzer T, et al. JAMA. 2019;322(1):37-48.
2. Berenbaum F, et al. Ann Rheum Dis. 2020;79(6):800-10.
3. Hochberg M, et al. Arthritis Rheumatol. In Press.
4. Sharma A, et al. Open Access Rheumatol. 2016;31(8):103-13.
5. Mallen C, et al. PLoS Med. 2017;14(4):e1002273.
6. Campbell C, et al. Arthritis Care Res. 2015;67(10):1387-96.
To cite this abstract in AMA style:
Mease P, Mallick-Searle T, Johnston E, Viktrup L, Menuet D, Yang R, Fountaine R. Efficacy of Subcutaneous Tanezumab for the Treatment of Osteoarthritis of the Knee or Hip: A Post Hoc Subgroup Analysis of Patients from a Randomized, NSAID-Controlled Study with a History of Depression, Anxiety, or Insomnia [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-subcutaneous-tanezumab-for-the-treatment-of-osteoarthritis-of-the-knee-or-hip-a-post-hoc-subgroup-analysis-of-patients-from-a-randomized-nsaid-controlled-study-with-a-history-of-depressi/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-subcutaneous-tanezumab-for-the-treatment-of-osteoarthritis-of-the-knee-or-hip-a-post-hoc-subgroup-analysis-of-patients-from-a-randomized-nsaid-controlled-study-with-a-history-of-depressi/