Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Cardiac involvement in systemic sclerosis (SSc) is common, underdiagnosed, and manifests variably. Myocardial involvement can manifest as left heart failure with preserved or reduced ejection fraction (HfpEF or HfrEF), right heart failure, restrictive cardiomyopathy, or myocarditis., often leading to a poor prognosis and reduced survival. Sacubitril/valsartan (SV), an angiotensin receptor neprilysin inhibitor, works by increasing the levels of natriuretic peptides and activating several signaling pathways that promote vasodilation, natriuresis, and the inhibition of the renin-angiotensin and sympathetic systems. Our descriptive study aimed to determine the effects of SV in patients with SSc and heart failure.
Methods: A retrospective analysis was conducted utilizing an electronic data extraction tool to identify SSc patients who received SV treatment across Mayo Clinic sites from January 2015 to August 2023. Comprehensive clinical phenotyping was performed, and longitudinal data were analyzed to evaluate the impact of S/V on specified outcomes. Paired T-tests were utilized to compare baseline and follow-up values for continuous variables. Independent T-tests were applied to compare outcomes across different scleroderma phenotypes, and chi-square tests were used for categorical variables. Kaplan-Meier survival analysis was conducted to estimate hospitalization and survival rates
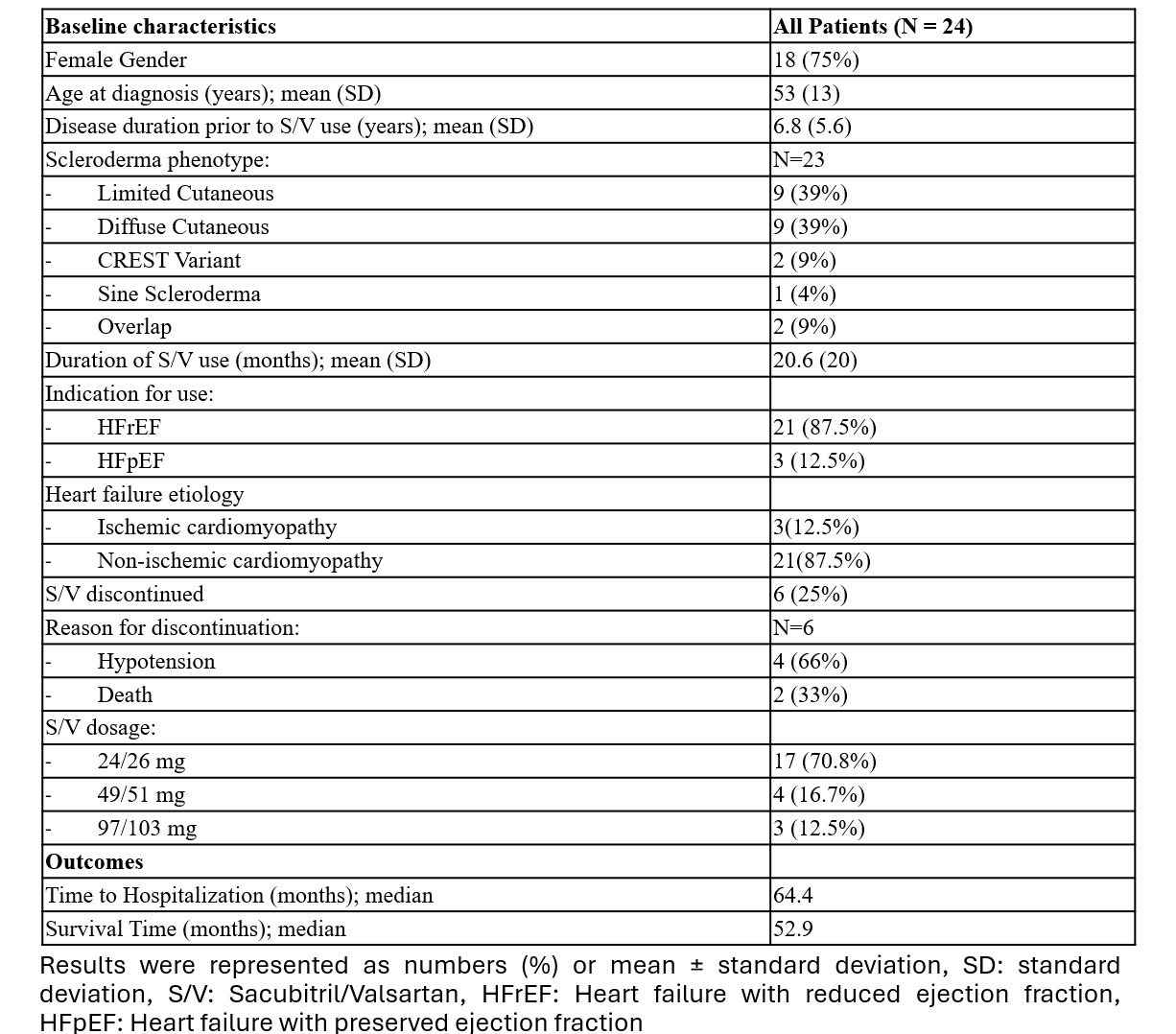
Results: A cohort of 24 patients with SSc undergoing treatment with SV was identified, with a female predominance of 75% and a mean age at diagnosis of 53 years. The average duration of SV therapy was 20.6 months. The primary indication for SV administration was HFrEF in 91% and the majority (87%) had non-ischemic cardiomyopathy.
Regarding the impact of SV on NYHA class in patients with Ssc and heart failure, at baseline, the distribution was: Class 1 (15%), Class 2 (35%), Class 3 (45%), and Class 4 (5%). At follow-up, the distribution shifted to: Class 1 (30%), Class 2 (45%), Class 3 (20%), and Class 4 (5%). The change was statistically significant (p = 0.04), suggesting improved heart failure symptoms with SV treatment.
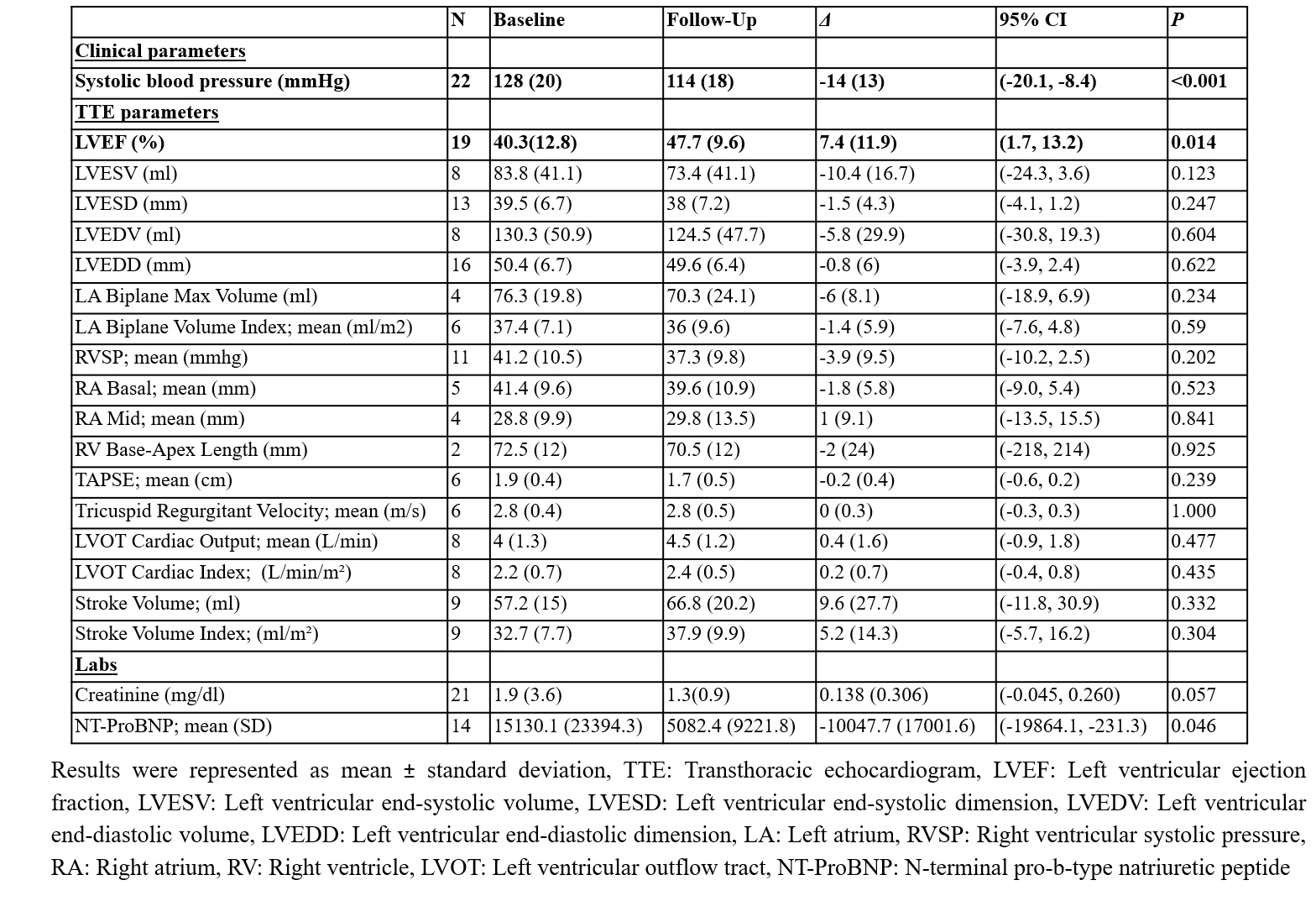
NT-ProBNP levels demonstrated a significant reduction from 15,130.1 pg/mL to 5,082.4 pg/mL (reduction of 10,047.7 pg/mL, p=0.046). A reduction in systolic blood pressure from 128 mmHg to 114 mmHg (reduction of 14 mmHg, p< 0.001) was observed. Additionally, left ventricular ejection fraction (LVEF) improved from 40.3% to 47.7% (increase of 7.4%, p=0.014). No significant differences in outcomes were observed between the diffuse and limited/CREST variants of SSc. SV was discontinued in 25% of the cohort, primarily due to hypotension (4) and mortality (2).
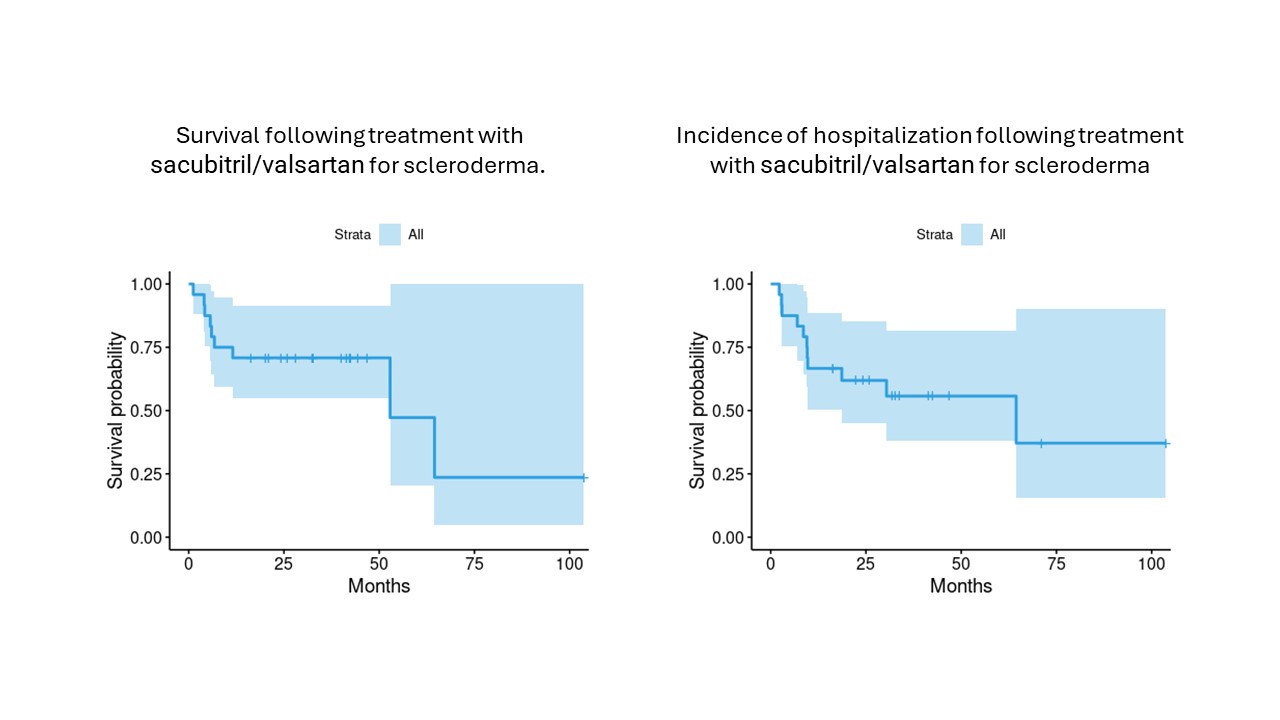
Kaplan-Meier survival analysis showed a median survival at 52.9 months, and median time to hospitalization of 64.4 months
Conclusion: In our small retrospective cohort of patients with SSc and heart failure, SV demonstrated improvements in NYHA class, cardiac function (including left ventricular ejection fraction), and reductions in NT-ProBNP levels. Future studies should focus on larger cohort with a comparator group to assess the impact on the trends in hospitalization and survival.
To cite this abstract in AMA style:
Eshak N, Abdelnabi M, Quillen J, Pham M, Hentz J, Nagaraja V. Efficacy of Sacubitril/Valsartan in Patients with Systemic Sclerosis and Heart Failure: A Retrospective Analysis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-sacubitril-valsartan-in-patients-with-systemic-sclerosis-and-heart-failure-a-retrospective-analysis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-sacubitril-valsartan-in-patients-with-systemic-sclerosis-and-heart-failure-a-retrospective-analysis/