Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Interleukin-6 (IL) plays an important role in the pathogenesis of comorbid rheumatoid arthritis (RA) depression, and IL-6 inhibitors used to treat RA patients may have antidepressant effects. The objective was to evaluate the efficacy of IL-6 inhibitor olokizumab (OKZ) in reducing the symptoms of depression in patients with moderate/high RA disease activity.
Methods: To date, 76 RA patients have been included, of which 65 (85.5%) are women, with an average age of 48.2±11.5 years; with predominantly high RA disease activity according to DAS28 (CRP) (89.2%), SDAI (83.8%) and CDAI (79.7%) indices and inefficacy of stable 12-week csDMARD therapy. In all patients, a psychiatrist diagnosed chronic or recurrent depression of varying severity (in accordance with ICD-10) during a semi-structured interview. At week 0, all patients were randomized by the method of sequential numbers in a ratio of 1:1:1 to one of the 3 study groups: group 1 – csDMARDs+OKZ 64 mg subcutaneously once every 4 weeks (n=27); group 2 – csDMARDs+OKZ 64 mg subcutaneously once every 4 weeks + psychopharmacotherapy (PPT) (n=39); group 3 – csDMARDs+PPT (n=10). The duration of the study is 24 weeks. The dynamics of depression severity were assessed by the Patient’s Health Questionnaire – 9 (PHQ-9), Montgomery – Asberg Depression Rating Scale(MADRS); anxiety – Hamilton Anxiety Rating Scale (HAM-A); experimental psychological projective tests were also used.
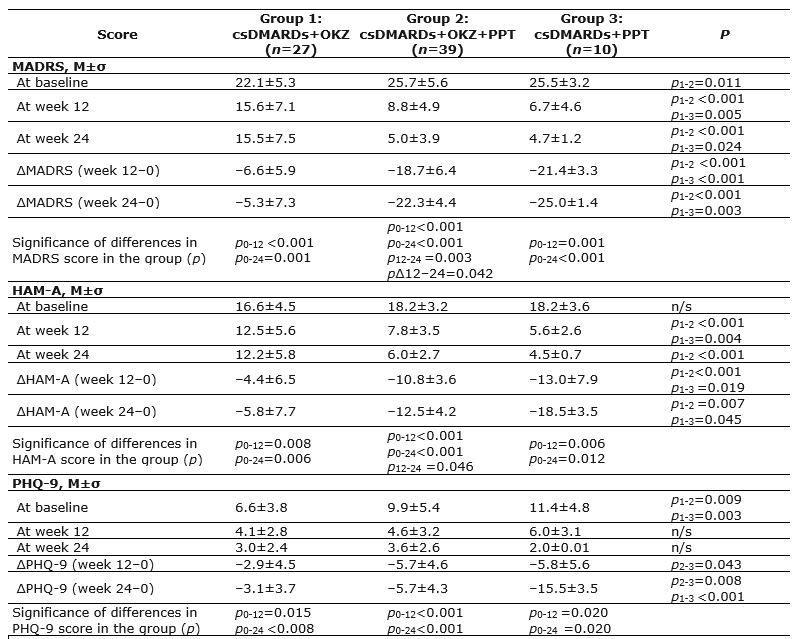
Results: There were no differences between the three groups in the duration of RA, severity of pain, TJC, SJC, nor patient or physician global assessments, physical function limitations according to the HAQ DI and health related quality of life according to EQ-5D-3L. After 12 and 24 weeks of therapy, there was a significant decrease in the severity of depression and anxiety in all groups of patients. However, the difference between the final and initial values of all scales was statistically significantly greater (p< 0.05) in the groups of patients receiving PPT: csDMARDs+PPT (ΔPHQ-9 24–0=–15.5±3.53; ΔMADRS 24–0=–25.0±1.41; ΔHAM-A 24-0=–18.5±3.53) and csDMARDs+OKZ+PPT (ΔPHQ-9 24–0=–5.73±4.31; ΔMADRS 24–0=–22.3±4.44; ΔHAM-A 24-0=–12.5±4.22), compared with the csDMARDs+OKZ group (ΔPHQ-9 24–0=–3.08±3.67; ΔMADRS 24-0=–5.25±7.33; ΔHAM-A 24–0=–5.75±7.74) (Table 1). According to a semi-structured psychiatric interview and experimental psychological tests, the proportion of patients without depression after 24 weeks of therapy was significantly higher in the groups of patients receiving PPT: 100% in the group of csDMARDs+PPT and 83% – csDMARDs+OKZ+PPT, as opposed to 22% in the group of csDMARDs+OKZ. OKZ therapy contributed to the normalization of night sleep but did not lead to a decrease in the frequency and severity of cognitive impairments (CI).
Conclusion: OKZ has an antidepressant effect, leads to a decrease in the frequency of sleep disorders, but a complete regression of depression was also found in 22% of patients who received OKZ without PPT, mainly in patients with mild depression. The combination of OKZ and PPT was optimal for the complete regression of depression, anxiety and a decrease in the frequency and severity of CI.
Footnotes: csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; OKZ, olokizumab; PPT, psychopharmacological treatment; p, significance of inter-group differences; MADRS, Montgomery – Asberg Depression Rating Scale; HAM-A, Hamilton Anxiety Rating Scale; PHQ-9, Patient’s Health Questionnaire; n/s, inter-group differences were not significant.
To cite this abstract in AMA style:
Strand V, Lisitsyna T, Abramkin A, Veltishchev D, Seravina O, Kovalevskaya O, Borisova A, Kuzkina S, Ignatiev V, NASONOV E. Efficacy of Olokizumab in Comorbid Depressive Disorder in Patients with Rheumatoid Arthritis: Preliminary Results of a Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-olokizumab-in-comorbid-depressive-disorder-in-patients-with-rheumatoid-arthritis-preliminary-results-of-a-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-olokizumab-in-comorbid-depressive-disorder-in-patients-with-rheumatoid-arthritis-preliminary-results-of-a-study/