Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients (pts) with PsA and an inadequate response (IR) to conventional synthetic DMARDs are routinely treated with TNF inhibitors (TNFi).1,2 Intolerance/IR to TNFi may require switching treatments.1,2 Tofacitinib is an oral Janus kinase inhibitor for the treatment of PsA. Data on whether treatment can be safely and effectively switched from TNFi to tofacitinib are limited. This post hoc analysis assessed tofacitinib efficacy and safety in pts with PsA in a long-term extension (LTE) study who had received either adalimumab (ADA) or tofacitinib in a Phase (P)3 study.
Methods: Data were analyzed frompts with active PsA who received tofacitinib 5 mg twice daily (BID) or ADA 40 mg once every 2 weeks in aP3 randomized, double-blind, placebo-controlled 12-month study (NCT01877668; OPAL Broaden) and then continued or switched to tofacitinib 5 mg BID and maintained this dose in an open-label LTE study (NCT01976364; OPAL Balance). Efficacy outcomes were assessed 3 months before the last visit and at the last visit/Month (M)12 in the P3 study, and at M3 (or M6 for select outcomes) in the LTE study and included: proportions of pts achieving ACR20, Psoriasis Area and Severity Index 75 (PASI75), HAQ-Disability Index (DI) response (decrease from baseline ≥ 0.35 for pts with baseline HAQ-DI ≥ 0.35), Psoriatic Arthritis Disease Activity Score (PASDAS) ≤ 3.2, minimal disease activity (MDA); and least squares mean change from baseline (Δ) in Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F). Safety outcomes were assessed at 3-month intervals to M12 in both studies and included proportions and incidence rates (pts with events/100 pt-years) for: treatment-emergent adverse events (TEAEs), serious AEs, and serious infections. Data reported as observed.
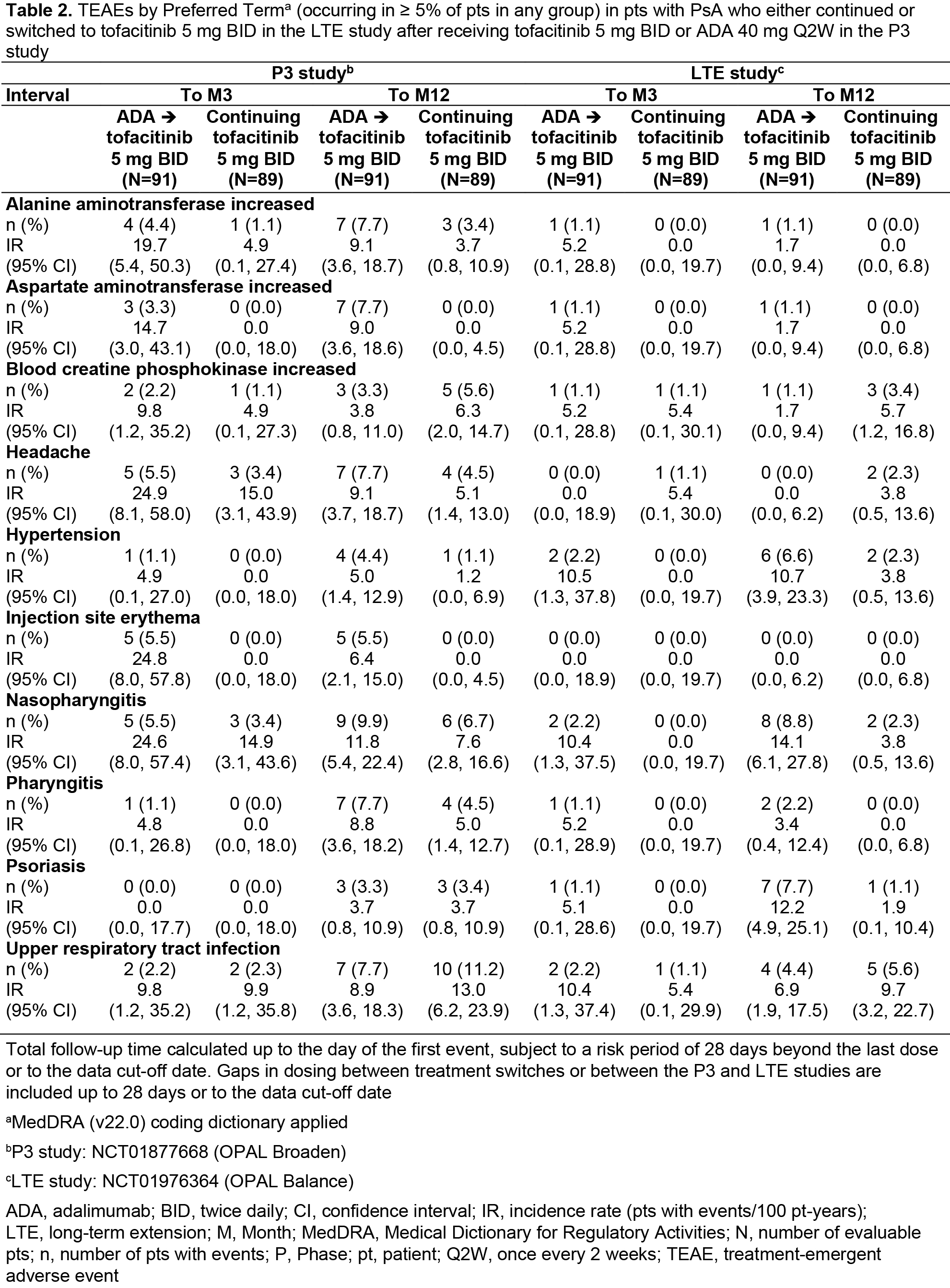
Results: In this post hoc analysis, 180 pts from the P3 study received tofacitinib 5 mg BID in the LTE study (ADAàtofacitinib 5 mg BID: n=91, 50.6%; continuing tofacitinib 5 mg BID: n=89, 49.4%). Baseline characteristics were generally similar across groups. Approximately half of pts were female (50.6%), with a mean (standard deviation [SD]) age of 47.5 (11.3) years and mean (SD) PsA disease duration of 5.9 (6.2) years. Efficacy was similar between groups 3 months before the last visit and at the last visit in the P3 study and was maintained to M3 (or M6 for PASDAS ≤ 3.2 and ΔFACIT-F) in the LTE study (Fig a–f). TEAEs, serious AEs, and serious infections were similar in the P3 and LTE studies, and between groups within each study (Table 1). The most common TEAEs across both studies were nasopharyngitis and upper respiratory tract infection (Table 2).
Conclusion: Tofacitinib efficacy and safety were similar in pts with PsA who continued or switched to tofacitinib 5 mg BID in the LTE study after receiving tofacitinib 5 mg BID or ADA in the P3 study, respectively. These data suggest that treatment can be directly switched from ADA to tofacitinib 5 mg BID. Limitations include the post hoc nature, small sample size and short time frame analyzed.
- Gossec et al. Ann Rheum Dis 2016; 75: 499–510
- Coates et al. Arthritis Rheumatol 2016; 68: 1060–71
Study sponsored by Pfizer. Medical writing support provided by S Gill, CMC Connect; funded by Pfizer.
To cite this abstract in AMA style:
Gladman D, Nash P, Mease P, FitzGerald O, Masri K, Duench S, Cadatal M. Efficacy and Safety of Tofacitinib in an Open-Label, Long-Term Extension Study in Patients with Psoriatic Arthritis Who Received Adalimumab or Tofacitinib in a Phase 3 Randomized Controlled Study: A Post Hoc Analysis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-tofacitinib-in-an-open-label-long-term-extension-study-in-patients-with-psoriatic-arthritis-who-received-adalimumab-or-tofacitinib-in-a-phase-3-randomized-controlled-study-a-p/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-tofacitinib-in-an-open-label-long-term-extension-study-in-patients-with-psoriatic-arthritis-who-received-adalimumab-or-tofacitinib-in-a-phase-3-randomized-controlled-study-a-p/