Session Information
Session Type: ACR Late-breaking Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Sprifermin, a novel recombinant human fibroblast growth factor-18 is currently investigated as a potential disease-modifying osteoarthritis (OA) drug. Two-year primary data from a 5-year Phase II trial (FORWARD) is presented.
Methods: Patients (pts) aged 40–85 years with symptomatic radiographic knee OA, KLG 2 or 3, and medial mJSW ≥2.5 mm in the target knee were randomized (1:1:1:1:1) to receive 3 weekly intra-articular injections with double-blinded placebo (PBO) or sprifermin, administered in cycles every 6 or 12 months (Fig 1). The primary endpoint was the change in total tibiofemoral joint (TFJ) cartilage thickness from baseline (BL) to Year 2. The ITT population (all randomized pts) was used for non-MRI endpoints; and the mITT (all ITT pts with BL and ≥1 post-treatment MRI up to Year 2) for MRI endpoints.
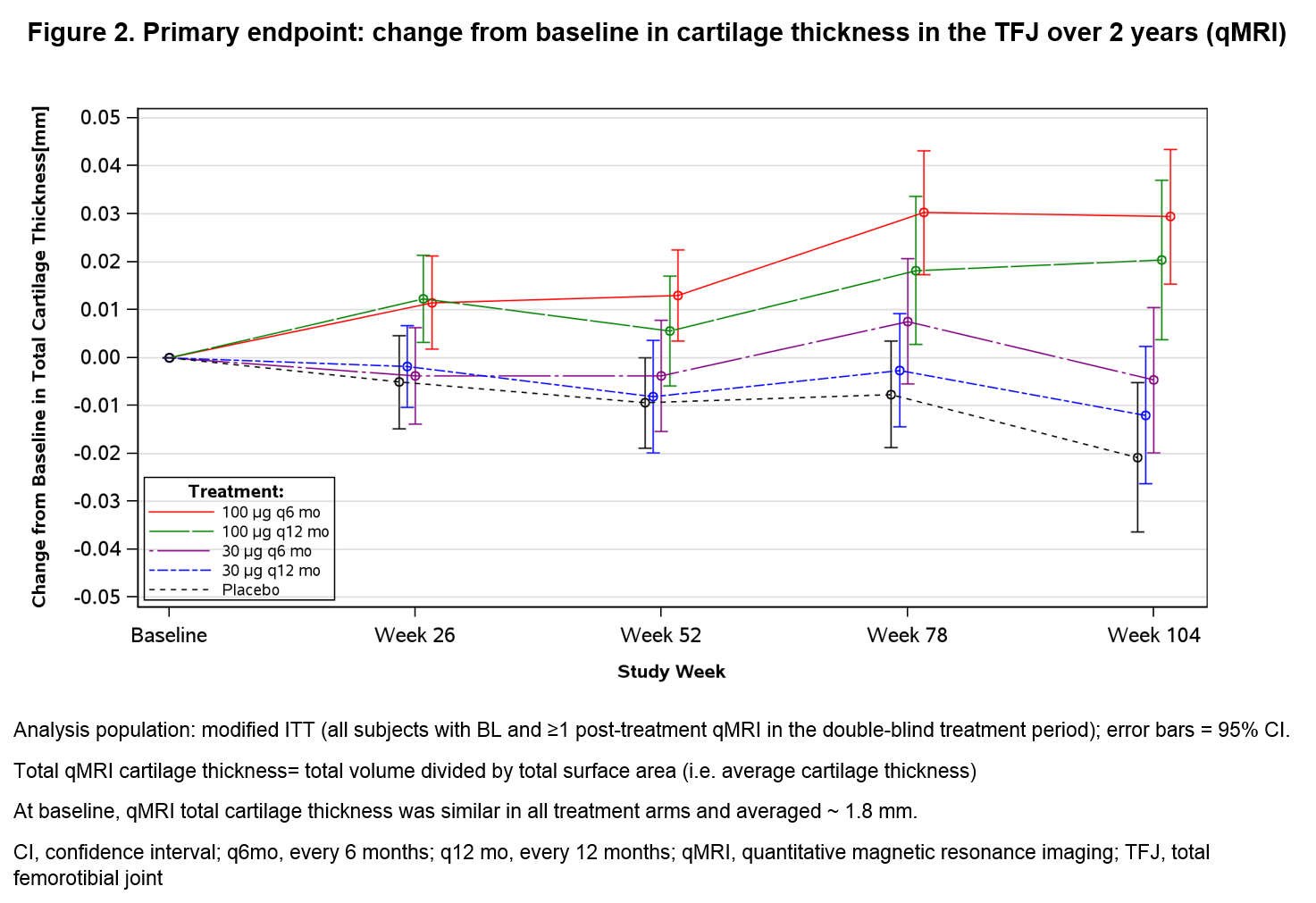
Results: The ITT population included 549 pts: median age 65 years, 69% women, 80% white, and 69% KLG2. Of these, 12.2% (sprifermin) and 19.4% (PBO) discontinued treatment within 2 years. The primary endpoint was met (Fig 2); there was a dose-dependent increase in total TFJ cartilage thickness, with significant differences for sprifermin 100µg q6 mo (Group 1) and 100µg q12 mo (Group 2) vs PBO (+0.03 vs -0.02 mm; p<0.001, and +0.02 vs -0.02 mm; p<0.001, respectively). Furthermore, significant differences in change of cartilage thickness were observed between sprifermin vs PBO in medial (Group 1: +0.02 vs -0.03 mm; p=0.003) and lateral TFJ compartments (Group 1 and 2: both +0.04 vs -0.01 mm; p<0.001), and in central medial TFJ sub-regions (Group 1: +0.054 vs -0.11; p<0.001). Changes in mJSW observed with X-ray were significantly different between sprifermin Group 1 and PBO in the lateral but not the medial compartment. Total WOMAC scores improved by ~50% in all treatment groups including PBO. AEs and serious AEs were balanced between groups, and an overall acceptable safety profile was observed.
Conclusion: To our knowledge, sprifermin is the first investigational agent to show prevention of cartilage loss in both the lateral and medial (including central medial) femorotibial compartments. These structural benefits associated with sprifermin suggest that it may be efficacious for disease-modifying treatment of OA, and should be further evaluated in clinical trials.
Disclosure: M. C. Hochberg, Bioiberica SA, Bristol Myers Squibb, EMD Serono, Galapagos, IBSA SA, Eli Lilly, Novartis Pharma AG, Pfizer Inc., Plexxikon, Samumed LLC, Theralogix LLC and TissueGene, 5; A. Guermazi, BICL, LLC, 1,Merck Serono, TissueGene, OrthoTrophix, AstraZeneca and Genzyme, 5; H. Guehring, Merck KGaA, 3; A. Aydemir, EMD Serono, Inc, 3; S. Wax, EMD Serono, Inc., 3; P. Fleuranceau-Morel, EMD Serono, Inc, 3; A. R. Bihlet, None; I. Byrjalsen, None; J. R. Andersen, None; F. Eckstein, Chondrometrics GmbH, 1.
To cite this abstract in AMA style:
Hochberg MC, Guermazi A, Guehring H, Aydemir A, Wax S, Fleuranceau-Morel P, Bihlet AR, Byrjalsen I, Andersen JR, Eckstein F. Efficacy and Safety of Intra-Articular Sprifermin in Symptomatic Radiographic Knee Osteoarthritis: Results of the 2-Year Primary Analysis from a 5-Year Randomised, Placebo-Controlled, Phase II Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-intra-articular-sprifermin-in-symptomatic-radiographic-knee-osteoarthritis-results-of-the-2-year-primary-analysis-from-a-5-year-randomised-placebo-controlled-phase-ii-study/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-intra-articular-sprifermin-in-symptomatic-radiographic-knee-osteoarthritis-results-of-the-2-year-primary-analysis-from-a-5-year-randomised-placebo-controlled-phase-ii-study/