Session Information
Date: Sunday, October 26, 2025
Title: Abstracts: Systemic Sclerosis & Related Disorders – Clinical I (0843–0848)
Session Type: Abstract Session
Session Time: 3:00PM-3:15PM
Background/Purpose: BMS-986353 (CC-97540) is an investigational CD19-directed T-cell therapy expressing the chimeric antigen receptor (CAR) used in globally-approved lisocabtagene maraleucel; it is manufactured via the NEX-T™ process to shorten manufacturing time and optimize phenotypic attributes. Early data from Breakfree-1 trial (NCT05869955) in severe, refractory autoimmune diseases (ADs) showed promising efficacy and manageable safety with BMS-986353.1 Here, we report updated data on BMS-986353 in the SSc cohort.
Methods: This phase 1 study assesses safety and efficacy in patients (pts) with severe, refractory ADs, including recently progressing diffuse SSc or limited SSc with interstitial lung disease (ILD) and inadequate response to ≥ 1 immunosuppressant. All SSc-directed therapies were discontinued before BMS-986353 infusion. Pts received a single infusion of BMS-986353 at 10×106 (dose level [DL] 1) or 25×106 (DL2) CAR+ T cells after lymphodepletion. Primary endpoint was safety.
Results: As of April 7, 2025, 14 pts with SSc were enrolled; 9 pts received BMS-986353 (DL1, n = 8; DL2, n = 1), with a median follow-up of 115.5 days (range 42–295) for DL1 and 168 days for DL2. Median age: 54.5 years for DL1, 41 years for DL2. Prior therapies: median 2 (range 1–5) for DL1 and 3 for DL2. At baseline, DL1 pts had a median modified Rodnan skin score (mRSS) of 18.0 and Physician’s Global Assessment (PGA) score of 7.0; scores for the DL2 pt were 7.0 and 4.0, respectively. ILD was present in 6 pts. Eight pts were efficacy evaluable (7 DL1, 1 DL2).All inflammatory AEs (iAE) reported were low grade. In DL1, 50.0% of pts had cytokine release syndrome (CRS; 3 grade 1, 1 grade 2) with a median duration of 2.0 days; 1 pt had grade 1 immune effector cell-associated neurotoxicity syndrome (ICANS) that resolved in 3 days (Table). There were 4 transient, self-resolving grade ≥ 3 hematologic TEAEs in DL1 pts; none in DL2. There were no prolonged grade ≥ 3 cytopenias, dose-limiting toxicities, or treatment-related infections.In DL1, median increase from baseline in absolute forced vital capacity in all patients was 3.0% on day 85 (n = 3) and 7.0% at day 169 (n = 3). In DL1, median reduction in mRSS across all pts with diffuse cutaneous SSc regardless of baseline mRSS was 14.0 points at day 85 (n = 3) and 11.5 at day 169 (n = 2) (Figure 1). Median reduction in PGA from baseline was 3.0 points on day 85 (DL1 n = 4; DL2 n = 1) and 4.5 on day 169 (DL1 n = 2). Improvement from baseline in revised Composite Response Index in Systemic Sclerosis was achieved in 3/3 pts at day 85 and day 169. All pts had robust CAR T cell expansion and achieved complete peripheral B-cell depletion (Figure 2).
Conclusion: BMS-986353 showed an acceptable safety profile and preliminary meaningful improvement in skin thickening and lung function in pts with severe, refractory SSc. iAEs were grade 1/2 and resolved quickly. Updated safety, efficacy, and translational data will be presented. Reference: 1. Schett G, et al. Arthritis Rheumatol 2024;76(suppl 9):1753.Medical writing: Joaquin Jaramillo, MD (Caudex, an IPG Health company), funded by Bristol Myers Squibb.
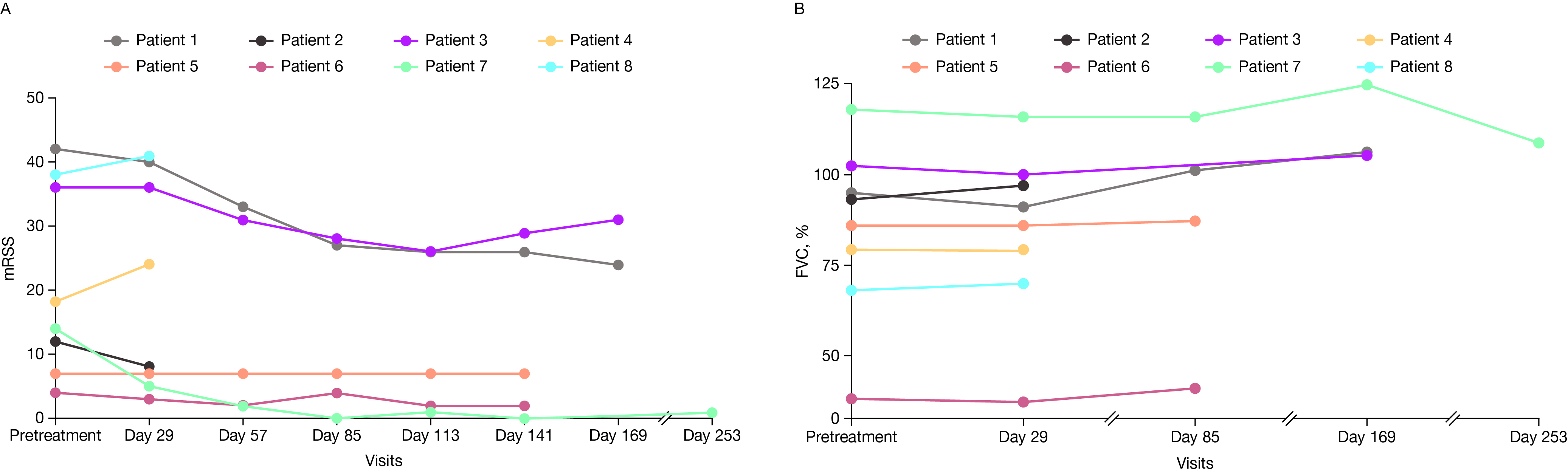 Table. CRS and ICANS by DL. a: Multiple events occurring within 7 days from each other are considered as 1 episode. CRS, cytokine release syndrome; DL, dose level; ICANS, immune effector cell-associated neurotoxicity syndrome.
Table. CRS and ICANS by DL. a: Multiple events occurring within 7 days from each other are considered as 1 episode. CRS, cytokine release syndrome; DL, dose level; ICANS, immune effector cell-associated neurotoxicity syndrome.
.jpg) Figure 1. (A) mRSS and (B) FVC in pts with SSc ≥ 1 month post-BMS-986353 at DL1 and DL2. FVC, forced vital capacity; mRSS, modified Rodnan skin score.
Figure 1. (A) mRSS and (B) FVC in pts with SSc ≥ 1 month post-BMS-986353 at DL1 and DL2. FVC, forced vital capacity; mRSS, modified Rodnan skin score.
.jpg) Figure 2. (A) Pharmacokinetic profile compared with lisocabtagene maraleucel and (B) B cell depletion. NHL, non-Hodgkin lymphoma; PK, pharmacokinetic; Q, quartile.
Figure 2. (A) Pharmacokinetic profile compared with lisocabtagene maraleucel and (B) B cell depletion. NHL, non-Hodgkin lymphoma; PK, pharmacokinetic; Q, quartile.
To cite this abstract in AMA style:
Khanna D, Korman D, Bernstein E, Kramer N, Majithia V, Mease P, Schett G, Azzi J, Nash R, Reshef R, Cherry M, Ayala E, Schwede M, Ghosh M, Müller F, Desai A, Ou S, Das S, Thorpe J, Harnois M, Melton A, Koegel A, Wiesendanger M. Efficacy and Safety of BMS-986353, a CD19-Directed Chimeric Antigen Receptor T Cell Therapy Manufactured Using a Next-Generation Process: Updated Data From a Phase 1 Trial in Patients With Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-bms-986353-a-cd19-directed-chimeric-antigen-receptor-t-cell-therapy-manufactured-using-a-next-generation-process-updated-data-from-a-phase-1-trial-in-patients-with-systemic-sc/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-bms-986353-a-cd19-directed-chimeric-antigen-receptor-t-cell-therapy-manufactured-using-a-next-generation-process-updated-data-from-a-phase-1-trial-in-patients-with-systemic-sc/
