Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Avacopan, an oral C5a receptor antagonist, offers a promising glucocorticoid (GC)-sparing option for patients with microscopic polyangiitis (MPA) and granulomatosis with polyangiitis (GPA). Although randomized trials have demonstrated its efficacy, real-world data, particularly in combination with rituximab-based induction, remain limited. Moreover, reliable biomarkers for monitoring avacopan treatment response are yet to be established. This study aimed to evaluate the effectiveness of avacopan and its impact on serum biomarkers—especially calprotectin—in MPA/GPA patients receiving rituximab for remission induction.
Methods: We retrospectively analyzed MPA/GPA patients who received rituximab-based induction therapy between January 2019 and December 2024, followed for at least 12 months. Patients were divided into those who received avacopan (avacopan group) and those who did not (non-avacopan group) during induction therapy. The primary endpoint was GC-free remission (defined as BVAS = 0 with complete GC withdrawal). Cumulative GC dose by month 12 was also assessed. In the avacopan group, serum calprotectin (S100A8/A9) and C5a levels were measured at baseline and month 3. Statistical comparisons between two groups were performed using the Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables. Paired t-tests were used to assess within-group changes in biomarkers. Pearson’s correlation coefficient was used to examine the relationship between changes in biomarkers and BVAS.
Results: A total of 47 patients were included (avacopan group: n = 21; non-avacopan group: n = 26). Baseline characteristics are shown in Figure 1. GC-free remission rate was significantly higher in the avacopan group at month 6 (19.1% vs. 0%, p = 0.034) and month 12 (38.1% vs. 0%, p < 0.001) (Figure 2). Cumulative GC dose at month 12 was significantly lower in the avacopan group (2343.2 ± 1242.3 mg vs. 4037.7 ± 1208.7 mg, p < 0.001). In the avacopan group, serum calprotectin levels significantly declined from 8082.1 ± 3624.6 ng/mL to 4170.5 ± 2975.8 ng/mL at month 3 (p = 0.035), and this change strongly correlated with BVAS improvement (r = 0.702, p = 0.024). In contrast, no significant change was observed in serum C5a levels (Figure 3).
Conclusion: Among MPA/GPA patients receiving rituximab-based induction therapy, avacopan enabled significant GC dose reduction while achieving clinical remission. Serum calprotectin decreased in response to treatment and correlated with disease activity, highlighting its potential as a real-world biomarker for monitoring avacopan efficacy.
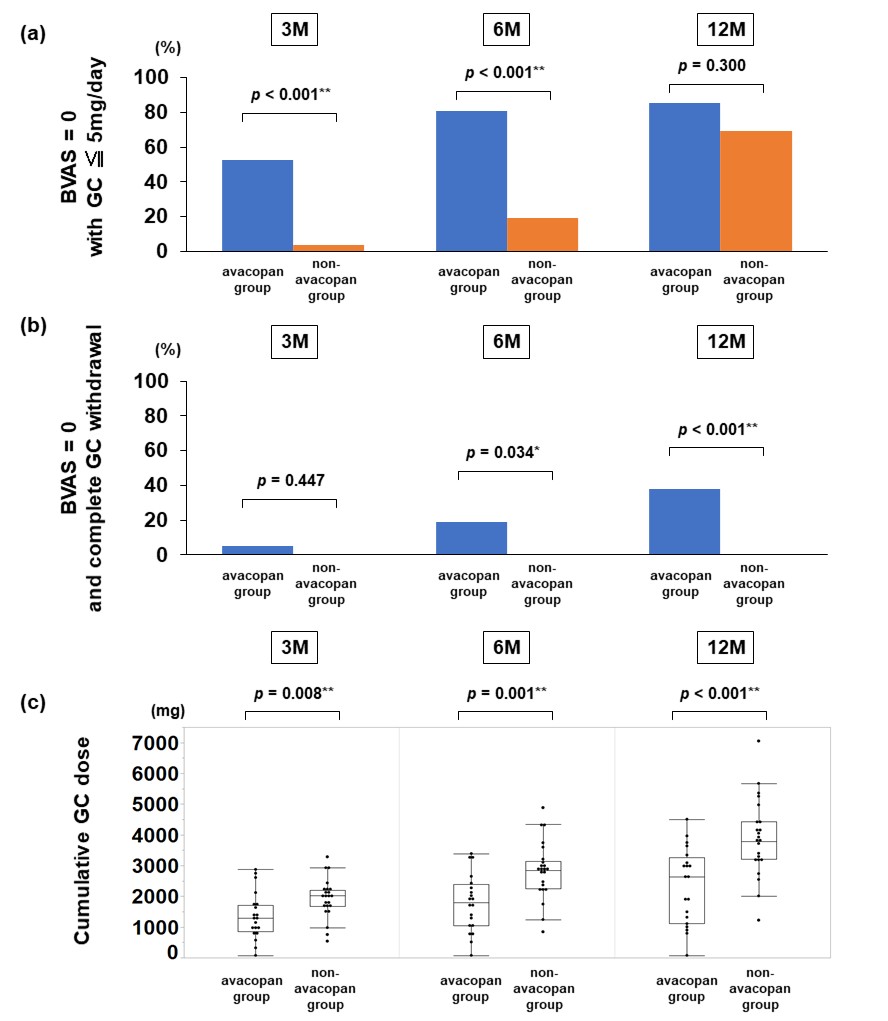 Figure 1. Baseline characteristics of the patients.
Figure 1. Baseline characteristics of the patients.
.jpg) Figure 2. Comparison of remission status and cumulative GC dose between the two groups. (a) Proportion of patients achieving BVAS = 0 with GC ≦ 5 mg/day. (b) Proportion of patients achieving BVAS = 0 and complete GC withdrawal (GC-free remission). (c) Cumulative GC dose through month 12.
Figure 2. Comparison of remission status and cumulative GC dose between the two groups. (a) Proportion of patients achieving BVAS = 0 with GC ≦ 5 mg/day. (b) Proportion of patients achieving BVAS = 0 and complete GC withdrawal (GC-free remission). (c) Cumulative GC dose through month 12.
.jpg) Figure 3. Serum biomarker changes and correlations with disease activity. (a) Changes in serum calprotectin and C5a levels from baseline to month 3. (b) Correlation between changes in BVAS and changes in serum calprotectin or C5a levels.
Figure 3. Serum biomarker changes and correlations with disease activity. (a) Changes in serum calprotectin and C5a levels from baseline to month 3. (b) Correlation between changes in BVAS and changes in serum calprotectin or C5a levels.
To cite this abstract in AMA style:
Ushio Y, Shimada H, Miyagi T, Sugihara K, Mizusaki M, Mino R, Chujo K, Manabe N, Wada M, Nakashima S, Dobashi H. Effectiveness of Avacopan and Its Impact on Serum Calprotectin in MPA/GPA Patients Receiving Rituximab-Based Remission Induction: A Single-Center Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-avacopan-and-its-impact-on-serum-calprotectin-in-mpa-gpa-patients-receiving-rituximab-based-remission-induction-a-single-center-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-avacopan-and-its-impact-on-serum-calprotectin-in-mpa-gpa-patients-receiving-rituximab-based-remission-induction-a-single-center-study/
