Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Interstitial lung diseases (ILD) are severe manifestations of systemic autoimmune diseases (SARD) that are associated with an increased mortality. Although the primary endpoint was not met, the focuSSed randomized controlled trial suggested that tocilizumab might preserve lung function in patients with systemic sclerosis (SSc) associated ILD. Similar results were observed in retrospective studies with small sample size for rheumatoid arthritis (RA) associated ILD. Our aim was to assess the effectiveness and tolerability of interleukin-6 inhibitors (IL6i) in SARD-ILD in a real-world cohort.
Methods: In this retrospective cohort study, we used an electronic query at a tertiary hospital to identify patients initiating an IL-6i with at least one code for ILD and one code for either SSc, RA, Sjogren Syndrome (SS), inflammatory myositis (IM), systemic lupus erythematosus (SLE), mixed connective tissue disease (MCTD) or undifferentiated connective tissue disease (UCTD). The diagnosis of SARD was verified by electronic medical record review and confirmed according to corresponding EULAR/ACR classification criteria. ILD diagnosis and chest high-resolution computed tomography (HRCT) scans pattern was performed by a multidisciplinary discussion meeting using clinical, functional, biological and radiological findings, including chest HRCT. We collected data regarding pulmonary function tests (PFT), chest HRCT evolution, and adverse events. A linear mixed model with random intercept was used to compare the within-patient trajectory of the percent predicted forced vital capacity (FVCpp) and percent predicted diffusing capacity of carbon monoxide (DLCOpp) within 18-months before to 18-months after IL6i introduction. Survival analyses were performed to asses IL6i retention.
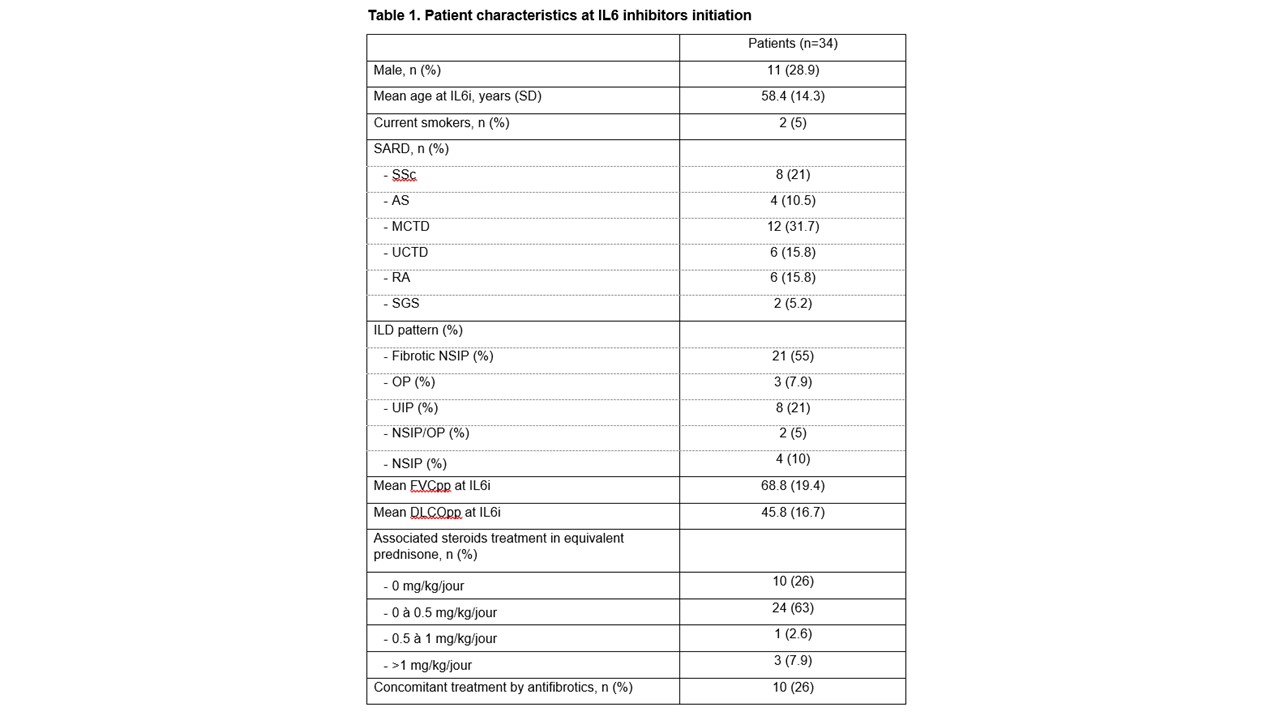
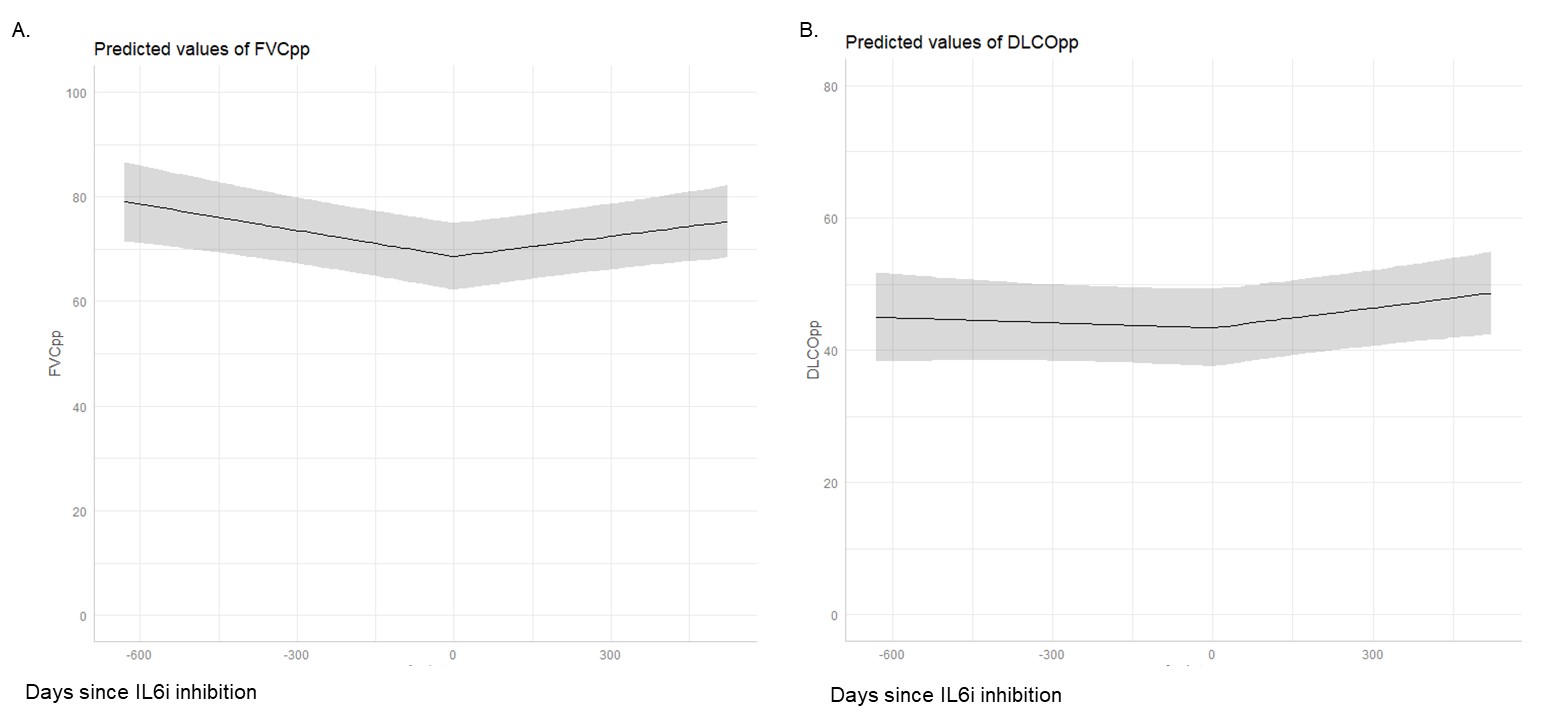
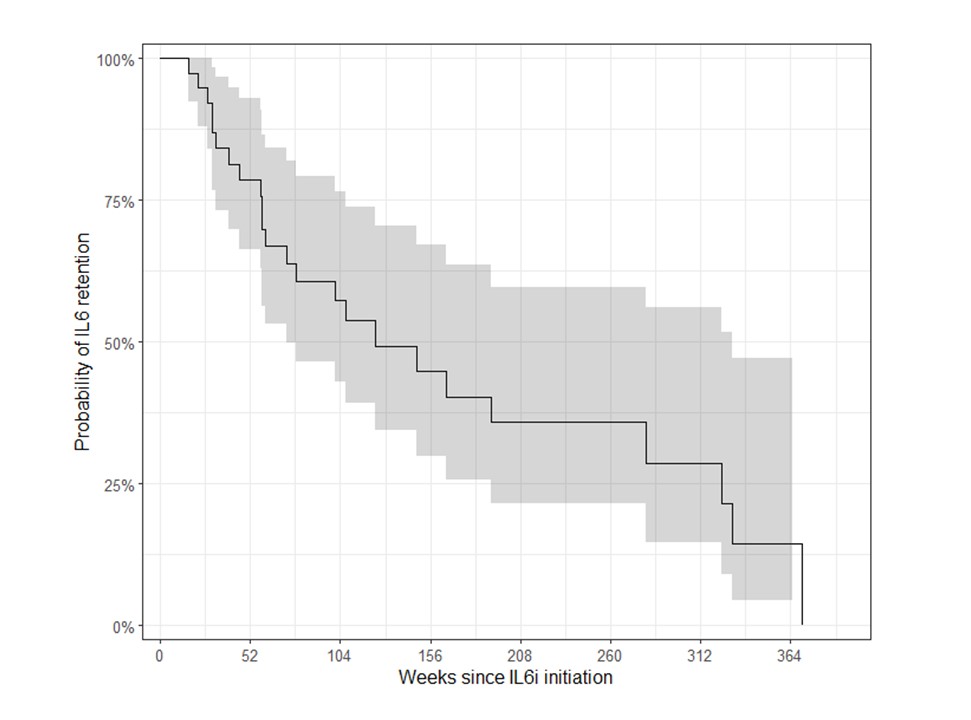
Results: We included 38 patients with SARD-ILD initiating an IL6i, (mean age 58.4 years, 11 males, 12 with MCTD, 8 with SSc, 6 with RA, 6 with UCTD, 4 with IM, and 2 with a primary SS, Table 1). Mean FVCpp and DLCOpp at IL6i initiation was 74.7 ± 19.4, and 45.8 ± 16.7, respectively. After IL6i initiation, we observed a significant change in the trajectory of FVCpp (+2.9% per year after IL6i initiation compared to -7.3% per year before, p = 0.002) and of DLCOpp (+4.7% per year prior to IL6i initiation vs. -0.17% per year, p = 0.02), Figure 1A. After a mean duration of 57.4 ± 26.1 weeks, qualitative clinical reports of ILD extension on chest HRCT scans showed that 19/34 patients had a stable ILD, 5/34 showed a regression of ILD, and 10/34 showed a progression of ILD. The median drug survival was 124.0 weeks (95% CI 73.1, 330), Figure 2. During the IL6i treatment, the following adverse events were reported in 8/38 patients: 3 severe infections, 2 non-severe infections, 2 cancers, 2 deaths and 1 transaminitis.
Conclusion: In this real-world study of IL6i in SARD-ILD, a significant improvement of lung function was observed after IL6i. In good agreement, about 2 third of the patients had a stable or an improvement of ILD extension.
To cite this abstract in AMA style:
Dupont A, Dupin C, Ebstein E, Forien M, Ottaviani S, Le Guen P, Philippot Q, Borie R, Crestani B, Dieudé P, Juge P. Effectiveness and Tolerability of Tocilizumab in Systemic Autoimmune Rheumatic Disease Associated Intertistial Lung Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/effectiveness-and-tolerability-of-tocilizumab-in-systemic-autoimmune-rheumatic-disease-associated-intertistial-lung-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-and-tolerability-of-tocilizumab-in-systemic-autoimmune-rheumatic-disease-associated-intertistial-lung-disease/