Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Children with rheumatic and autoimmune diseases are often treated with conventional or biologic disease-modifying antirheumatic drugs (cDMARDs and bDMARDs) to control disease. While effective, DMARDs may impact antibody responses to COVID-19 vaccination, leading to a risk of breakthrough infections. Here, we compared the effect of different treatments for rheumatic and autoimmune diseases on the antibody response to COVID-19 vaccination in children.
Methods: A prospective observational study was conducted across Canada. Serum or dried blood spots were collected from children with autoimmune or rheumatic diseases after each COVID-19 vaccine dose. Antibodies against the SARS-CoV-2 spike (SmT1) and receptor-binding domain (RBD) were measured using an automated ELISA platform and results are reported in binding antibody units per mL (BAU/mL). Children who had evidence of a SARS-CoV-2 infection prior to sample collection were excluded, except for the hybrid immunity analysis. The magnitude and kinetics of the antibody response after each vaccine dose were compared between treatment groups using mixed-effects linear regression models. Disease activity was measured in a subset of participants using the Childhood Health Assessment Questionnaire (CHAQ) and the Visual Analog Scale (VAS).
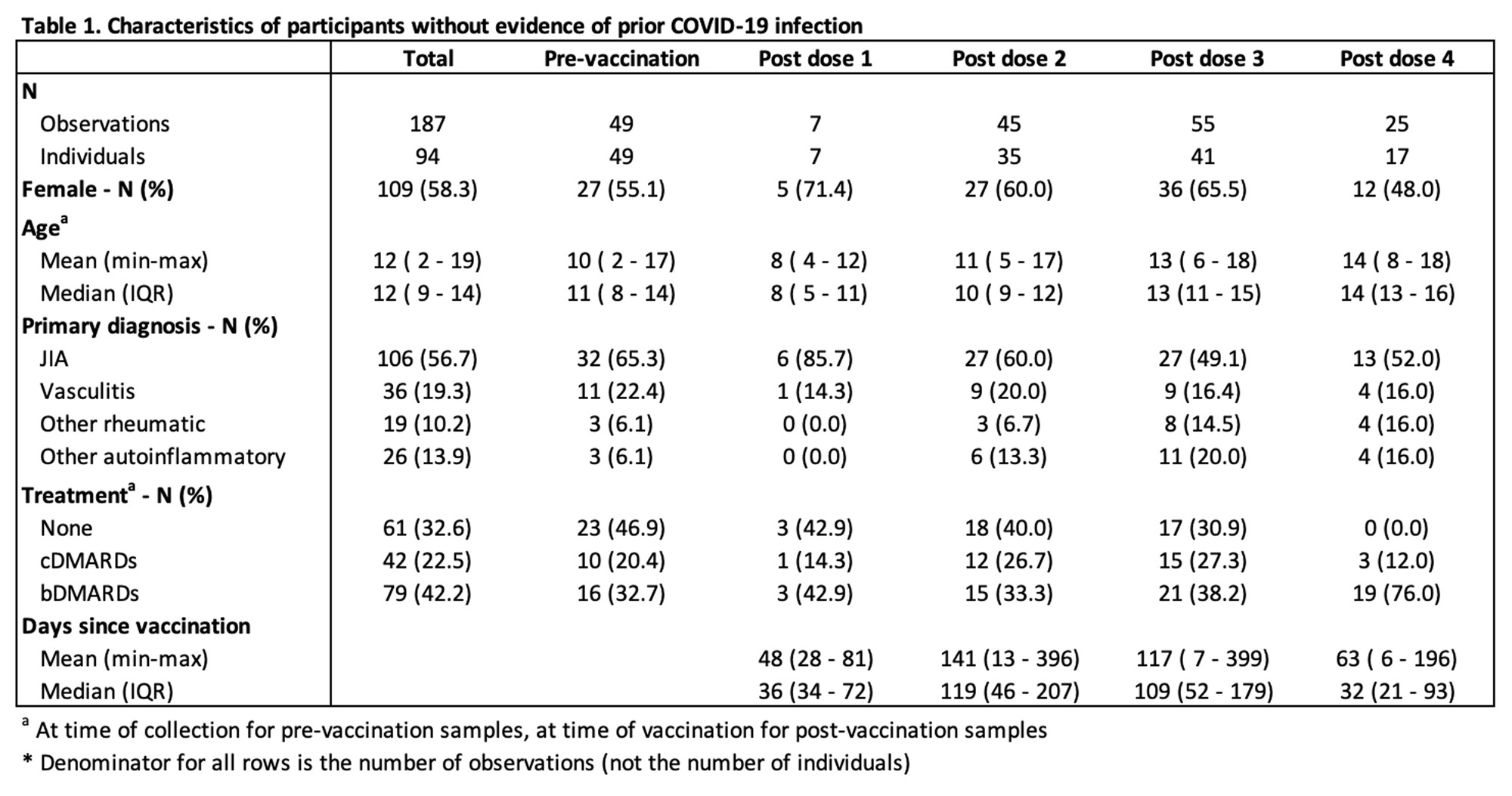
Results: For this interim analysis, 1751 potential participants were identified. Of these, 1748 were contacted and 192 enrolled in the study. Data are currently available for 126 participants, including from 94 individuals without evidence of prior COVID-19 infection. Participants contributed 1-6 samples, resulting in total of 187 samples collected pre-vaccination, and following 1-4 vaccine doses (Table 1). The median age of participants was 12 years and most patients had a primary diagnosis of either JIA (57%) or vasculitis (19%). There was a relatively even distribution between the three treatment groups (none, cDMARDs, and bDMARDs), with a slight over-representation of bDMARD-treated patients (42%).
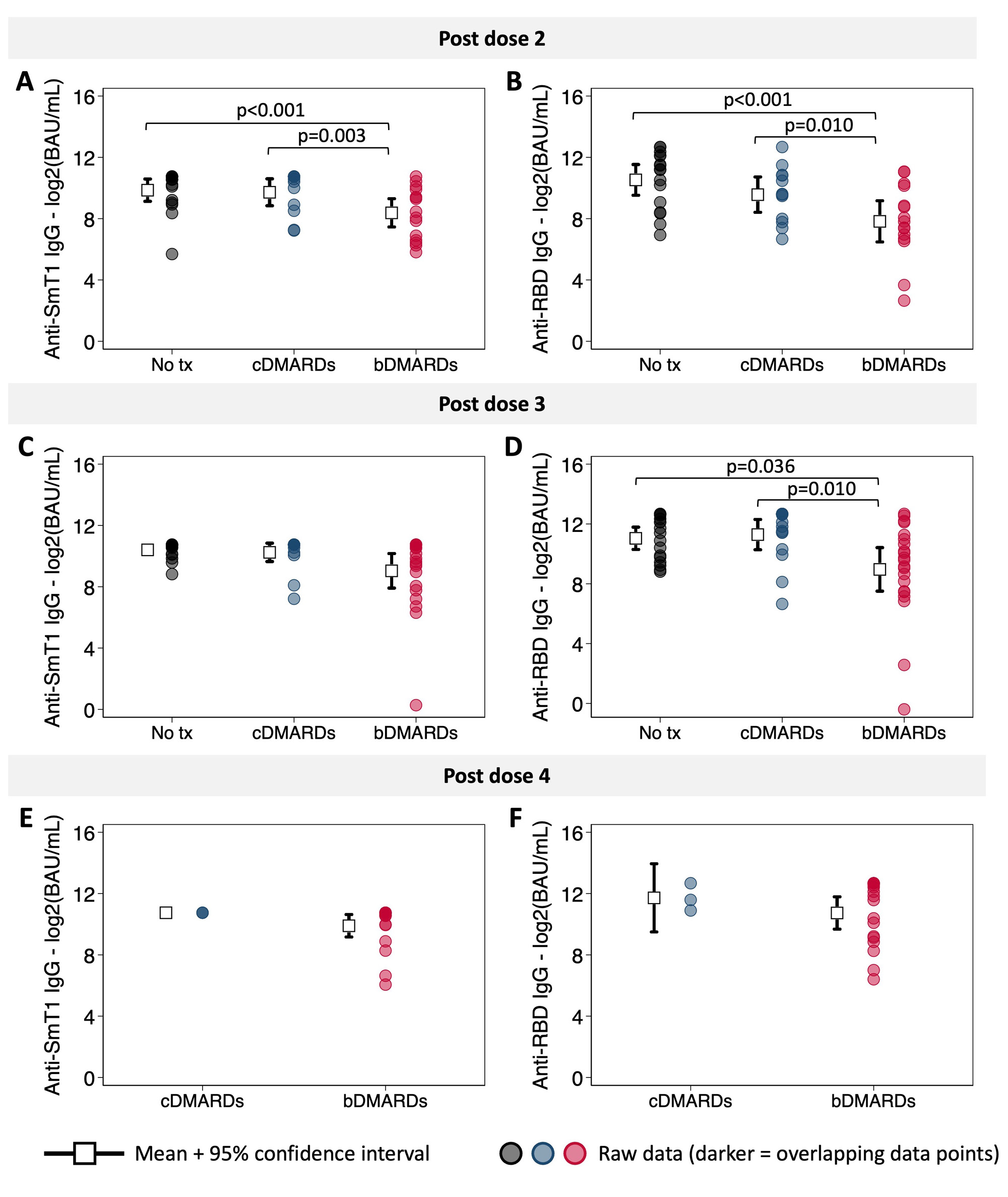
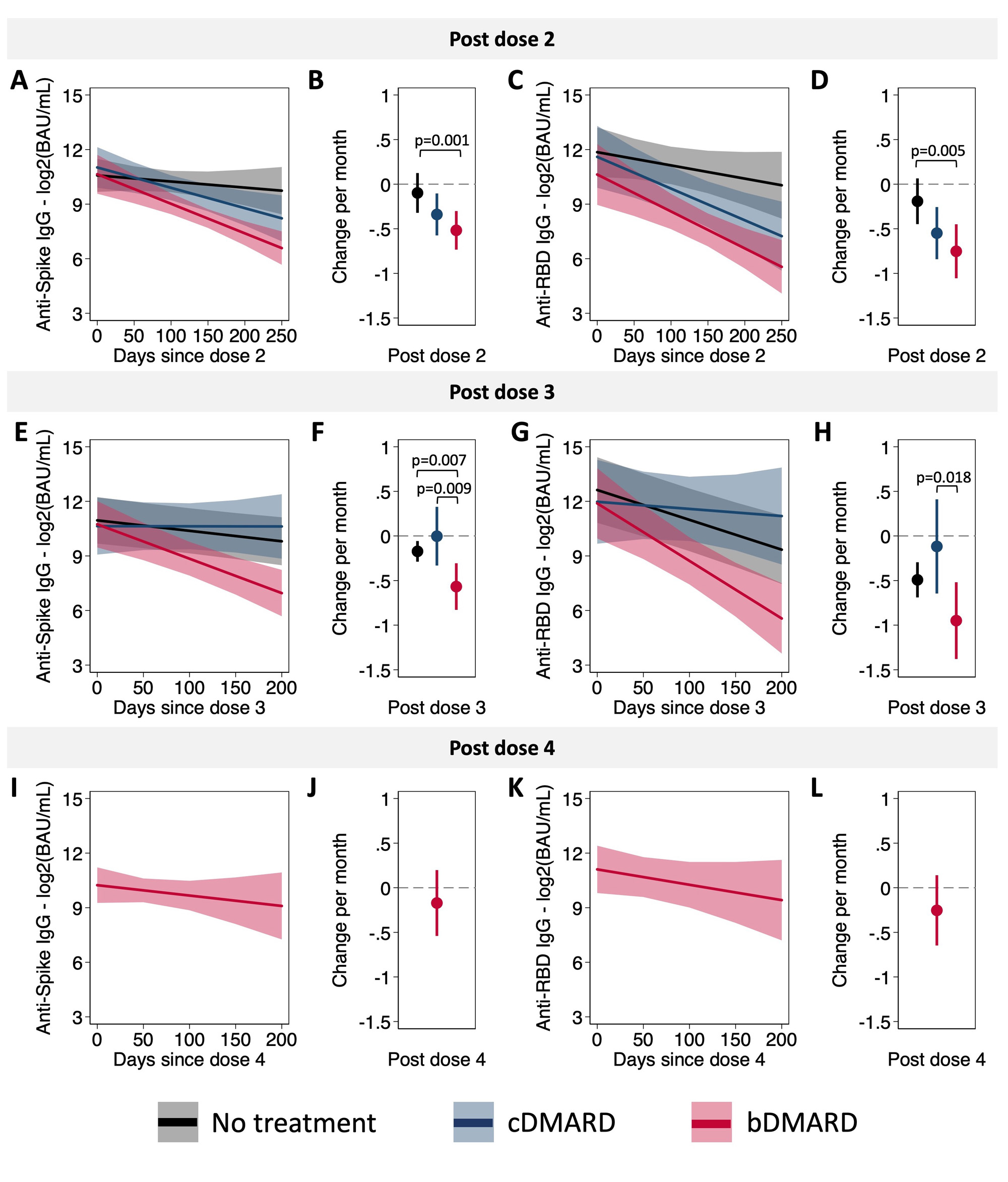
Following the second dose of the COVID-19 vaccine, IgG responses were both reduced in magnitude and waned faster for participants treated with bDMARDs than for those treated with cDMARDs or who were treatment naïve (Figures 1 & 2). Post-dose 3, differences in the magnitude of response between treatment groups were attenuated, but responses continued to wane at a faster rate in those treated with bDMARDs (Figures 1 & 2). Differences between treatment groups were not significant, however, among children with evidence of a prior SARS-CoV-2 infection. After dose 4, antibody responses were similar in all treatment groups (Figures 1 & 2). As measured by CHAQ and VAS scores reported by parents and participants, vaccination was not associated with increased disease activity.
Conclusion: Antibody responses to COVID-19 vaccination in children with autoimmune and rheumatic diseases were lowest in individuals treated with bDMARDs, with third and fourth vaccine doses necessary to yield robust and durable antibody responses. Given that vaccination did not impact underlying disease activity, additional work is needed to optimize vaccine strategies in this population.
To cite this abstract in AMA style:
Shapiro J, Choi F, Xu A, Duong T, Gingras A, Bernatsky S, Benseler S, Yeung R. Effect of Conventional and Biologic Disease-Modifying Anti-Rheumatic Drugs on the Antibody Response to Four Doses of COVID-19 mRNA Vaccines in Children with Autoimmune and Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/effect-of-conventional-and-biologic-disease-modifying-anti-rheumatic-drugs-on-the-antibody-response-to-four-doses-of-covid-19-mrna-vaccines-in-children-with-autoimmune-and-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-conventional-and-biologic-disease-modifying-anti-rheumatic-drugs-on-the-antibody-response-to-four-doses-of-covid-19-mrna-vaccines-in-children-with-autoimmune-and-rheumatic-diseases/