Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Citrullinated proteins are hallmark targets of the autoimmune response in rheumatoid arthritis (RA), but the mechanism by which immune tolerance is broken to these self-proteins is poorly understood. CD4+ T cells are implicated as important drivers of the autoimmune response due to the high-affinity, class-switched nature of anti-citrullinated protein antibodies (ACPAs) present in the majority of RA patients and the prominent genetic contribution of certain HLA-DR alleles to RA susceptibility. However, the precise effect of citrullination on MHC class II antigen processing and presentation of autoantigens to CD4+ T cells remains unknown. Here we aimed to examine the hypothesis that citrullination impacts the processing and presentation of RA autoantigens via destabilization of protein folding and modification of protease cleavage sites, altering the peptide repertoire presented by antigen-presenting cells (APCs).
Methods: Using fibrinogen as a model RA autoantigen, the native and citrullinated forms were digested in vitro by a cocktail of lysosomal cathepsins (cathepsins B, S, and H) for proteolytic mapping, or incubated with monocyte-derived dendritic cells (mo-DCs) in a natural antigen processing assay (NAPA). Peptides generated by digestion with the cathepsin cocktail or presented by HLA-DR molecules on mo-DCs were then isolated and identified by mass spectrometry.
Results: We found that the repertoire of peptides generated by each method was altered by citrullination. By proteolytic mapping, we detected both changes in the pattern of cathepsin cleavage and an increased number of peptides in the citrullinated samples. Utilizing NAPA, we observed the creation of newly presented peptides in the citrullinated samples in some cases, and loss of presented peptides in others (Fig. 1). Strikingly, all peptides whose presentation was destroyed by citrullination contained a citrullination site. Together these results suggest that both protease cleavage and selection of peptides by HLA-DR are impacted by citrullination.
Conclusion: Citrullination alters the peptide repertoire presented by APCs. Interestingly, no citrullinated peptides were identified by NAPA, suggesting that presentation of citrulline-containing peptides to T cells may not be the primary mechanism by which tolerance is broken to citrullinated antigens. Rather, citrullination-induced destabilization of protein folding and modification of protease cleavage sites, leading to the generation of a new peptide repertoire, could play a role in activating autoreactive T cells. This mechanism could thus drive the loss of immune tolerance to the citrullinated forms of RA autoantigens.
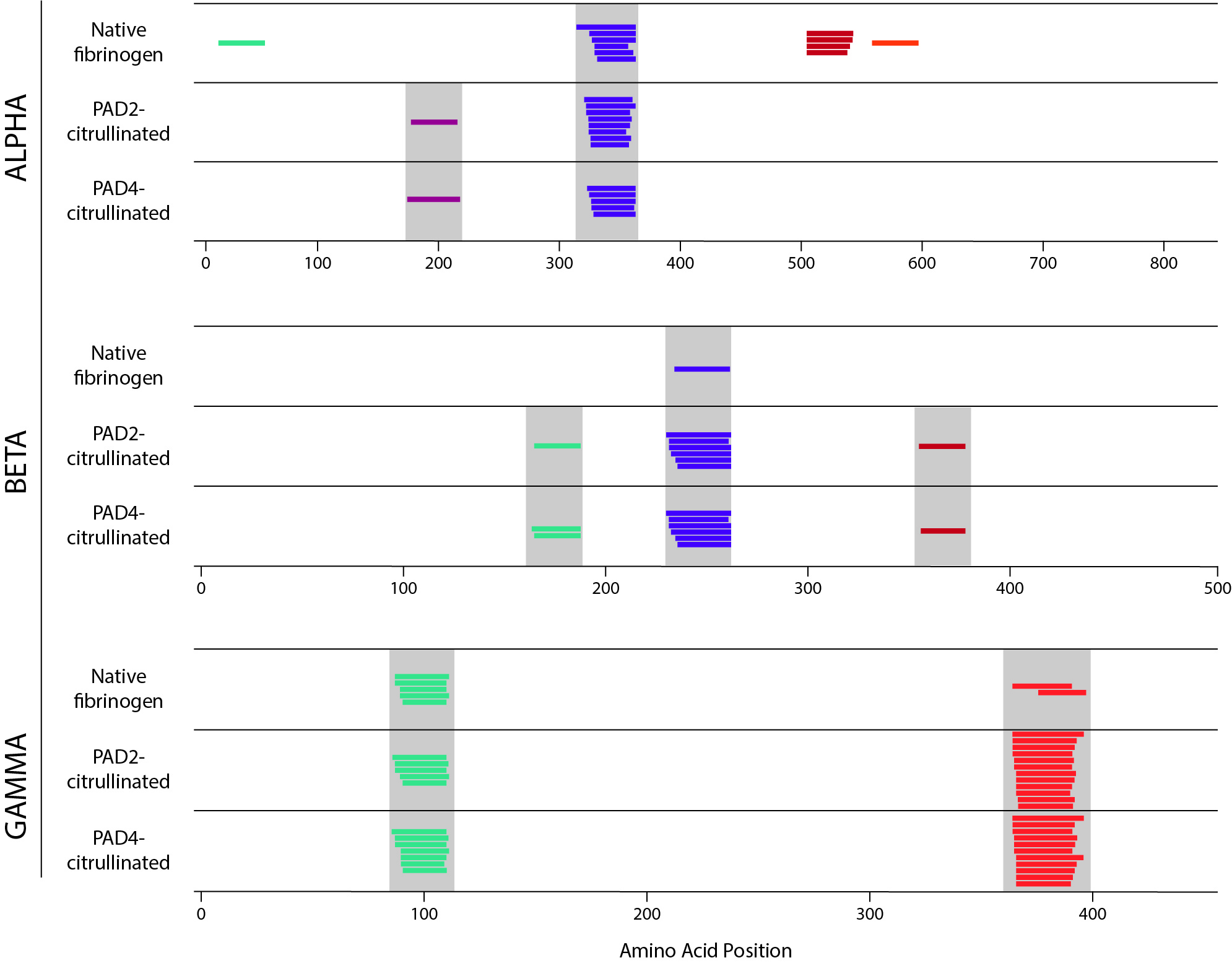 Figure 1. NAPA performed on healthy donor mo-DCs incubated with native, PAD2-citrullinated, and PAD4-citrullinated fibrinogen. Alpha, beta, and gamma chains of fibrinogen are shown separately. Each colored line represents a unique peptide. Nested peptides with a common core motif are shown in the same color within each chain. Grey bar denotes peptides with identical core motif between samples.
Figure 1. NAPA performed on healthy donor mo-DCs incubated with native, PAD2-citrullinated, and PAD4-citrullinated fibrinogen. Alpha, beta, and gamma chains of fibrinogen are shown separately. Each colored line represents a unique peptide. Nested peptides with a common core motif are shown in the same color within each chain. Grey bar denotes peptides with identical core motif between samples.
To cite this abstract in AMA style:
Curran A, Crawford J, Darrah E. Effect of Citrullination on the Processing and Presentation of Rheumatoid Arthritis Autoantigens [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/effect-of-citrullination-on-the-processing-and-presentation-of-rheumatoid-arthritis-autoantigens/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-citrullination-on-the-processing-and-presentation-of-rheumatoid-arthritis-autoantigens/
