Session Information
Date: Saturday, November 6, 2021
Title: RA – Diagnosis, Manifestations, & Outcomes Poster I: Cardiovascular Pulmonary Disease (0268–0295)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Cardiovascular disease (CVD) risk scores incorporating measures of inflammation such as the Reynolds risk score (RRS) may be appropriate to predict CVD risk in patients (pts) with rheumatoid arthritis (RA), even though not yet validated for RA pts. The purpose of this analysis was to investigate the effect of biologic DMARDs on lipids and the RRS.
Methods: Pts with at least moderate disease activity (CDAI >10) initiating a biologic DMARD participated in a comparative effectiveness trial (CERTAIN) nested within CorEvitas (formerly known as Corrona) RA registry. Characteristics, including lipid values, hsCRP and RRS in pts initiating a TNF-α inhibitor (TNFi) or non‐TNFi (rituximab [RTX], abatacept [ABT] or tocilizumab [TCZ]), were measured at baseline, 3- and 6- months later. Longitudinal mixed models examined the association of individual biologics with changes in lipid levels, hsCRP and RRS after 3- and 6-months of therapy. Log transformations used for skewed distributions (TG, hsCRP, RRS). Generalized structural equation models were estimated to model mediation of CRP, CDAI or swollen joint count on lipid changes when comparing TCZ vs other biologics. TCZ was selected for this comparison given the known – and observed in this analysis (see below) – effects on lipid levels. Patients who interrupted therapy prior to follow up visits or without complete lipid data at all time points were excluded.
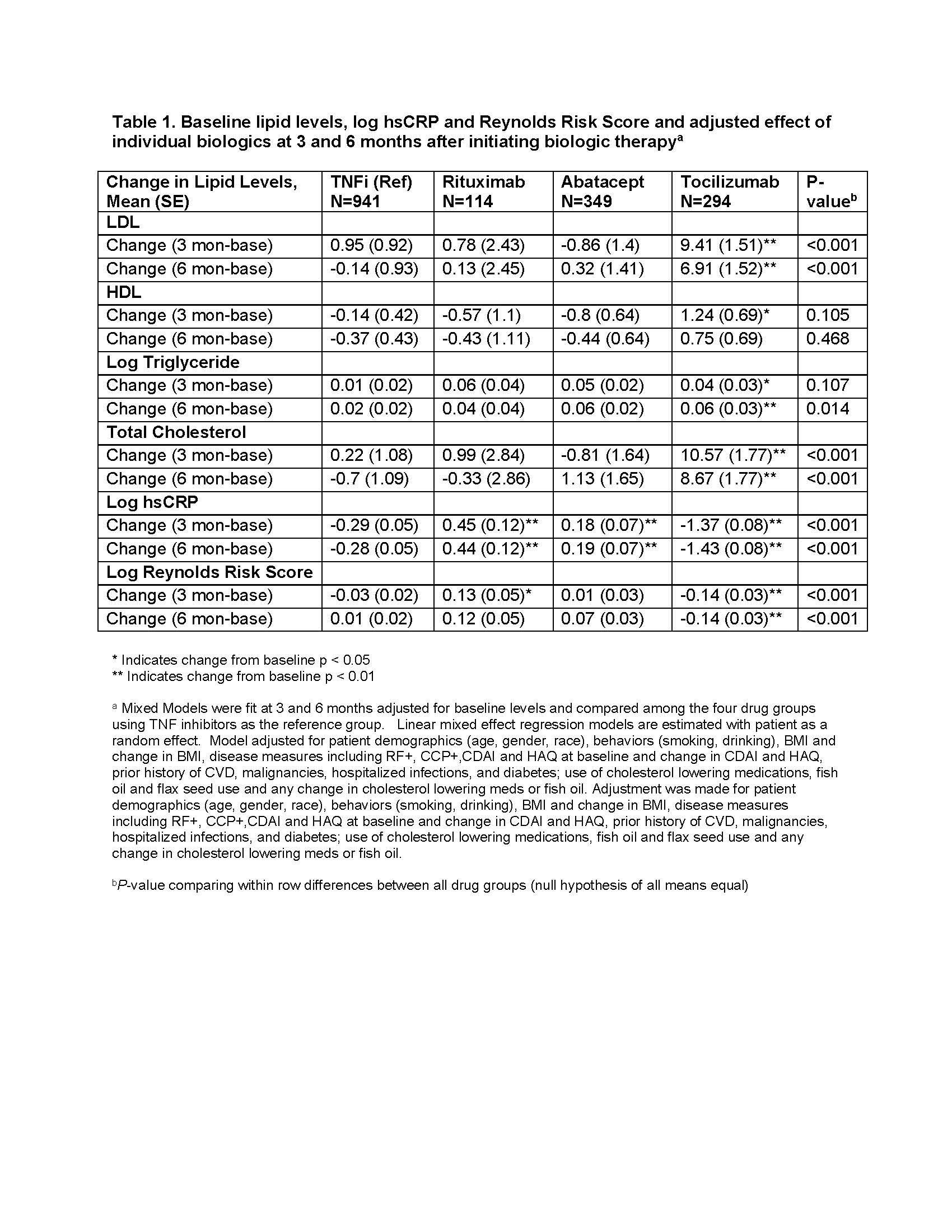
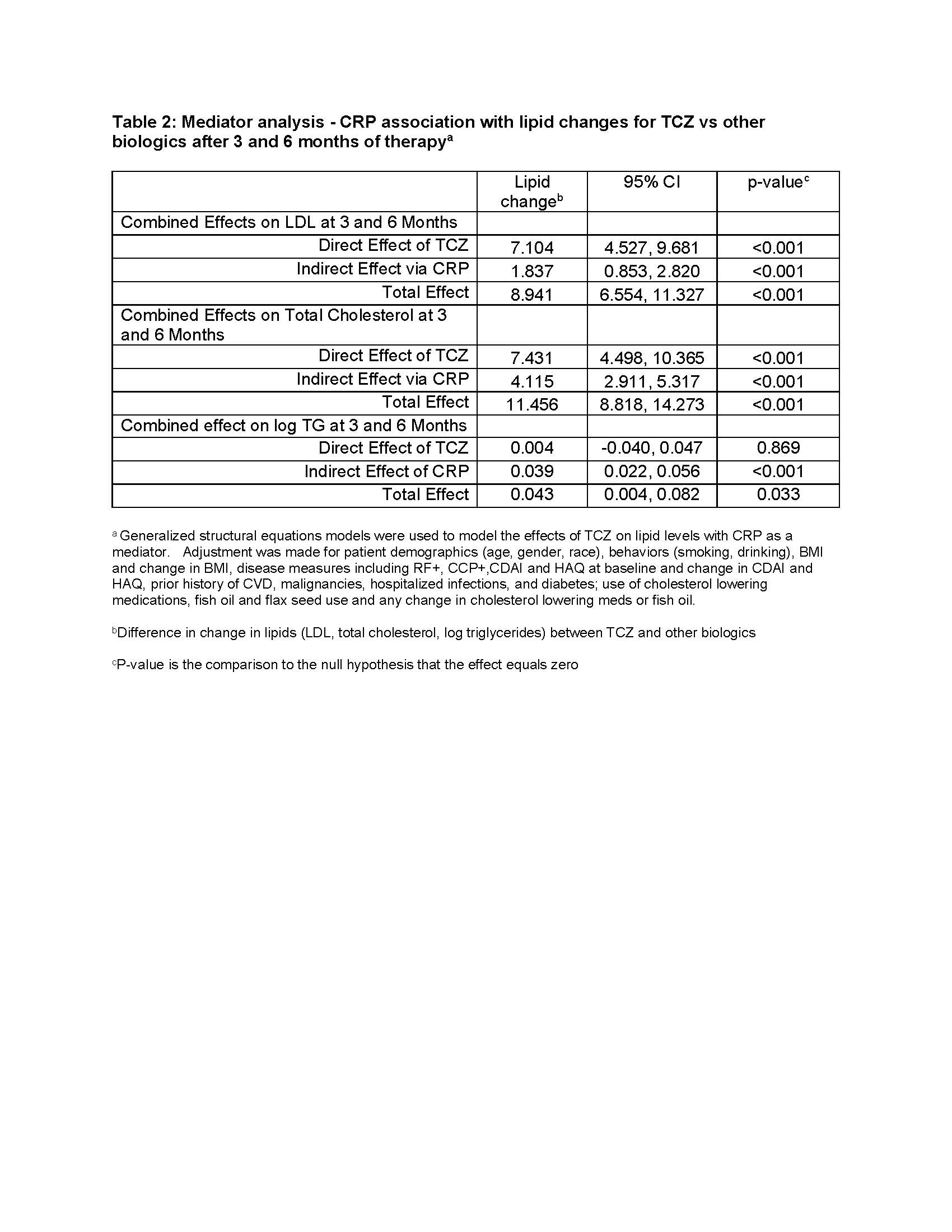
Results: 1698 initiations of a biologic were analyzed. Baseline characteristics: 78.7% women, 89.2% Caucasian, 72.3% seropositive. Mean ± SD age was 56.9 ± 12.8, RA disease duration 8.9 ± 9.2 years; CDAI 29.0 ± 12.7. 36.8% of pts were biologic naïve and 24.5% on anti-hyperlipidemic therapy. CVD history (7.9% of patients) did not exclude patients from the analysis. Diabetes mellitus was present in 9.4% of patients and 42.0% were obese (BMI >30). Table 1 shows lipid levels, hsCRP and RRS adjusted changes after 3- and 6-months of therapy. Pts initiating TCZ had a significant increase in total cholesterol, LDL, triglycerides (TG) and a significant decrease in hsCRP compared with patients initiating TNFi. Pts initiating ABT had a significant increase in hsCRP compared with patients initiating TNFi. ABT had significant decreases in log RRS at 3- and 6-month compared to other biologics. Mediator analyses estimated significant effect of hsCRP but not CDAI or swollen joints on lipid changes. Table 2 presents direct, indirect effects, and total effects of TCZ vs other biologics on lipid changes.
Conclusion: The impact of lipid changes on CVD risk must be considered in the context of the burden of inflammation in pts with RA. Lipid increases may not be atherogenic and may occur in the context of the “lipid paradox”. In this analysis moderate increases in lipid levels were associated with the therapy initiated and with changes in CRP. Lipid increase did not translate to an increased CVD risk as captured by RRS.
Refs: Ridker PM et al. (http://www.ncbi.nlm.nih.gov/pubmed/18997194) C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men.Circulation. 2008. 25;118(22):2243-51.
To cite this abstract in AMA style:
Pappas D, Reed G, Kane K, Curtis J, Kremer J. Effect of Biologic Agents on Lipids and Cardiovascular Risk in Rheumatoid Arthritis Patients [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effect-of-biologic-agents-on-lipids-and-cardiovascular-risk-in-rheumatoid-arthritis-patients-2/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-biologic-agents-on-lipids-and-cardiovascular-risk-in-rheumatoid-arthritis-patients-2/