Session Information
Date: Tuesday, October 28, 2025
Title: (2470–2503) Systemic Sclerosis & Related Disorders – Clinical Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic sclerosis (SSc) is characterized by skin fibrosis, dysregulated immune response, and vascular system dysfunction. Interstitial lung disease (ILD) is one of the leading causes of SSc-related deaths (Cottin 2019). SSc, associated with ILD, severely affects the immune system and contributes to skin and lung fibrosis. Hematopoietic stem cell transplantation (HSCT) works by resetting the immune system and is emerging as a potential therapeutic strategy for patients with severe SSc-ILD. However, in skin and in peripheral blood mononuclear cells (PBMC) of these patients, the molecular mechanisms and the cell populations that are impacted by the transplant are yet to be identified.
Methods: Single-cell RNA sequencing (scRNA-seq) and assay for transposase-accessible chromatin using sequencing (ATAC-seq) of skin biopsies and PBMCs were performed from four human healthy controls and three SSc-ILD patients before and one year after transplantation. The R packages Seurat (v4.3.0) and Signac (v1.11.0) were used to analyze the scRNA-seq and ATAC-seq data. Cell-cell communication analysis was performed using the R package CellChat (v2.1.2).
Results: Three patients (mean age 54 (range 37-63), 2 men and 1 woman) underwent myeloablative autologous stem cell transplant. The pre (mean 24, SD 5) and post one year (mean 6.33, SD 6.66) modified Rodnan skin score (mRSS) and pre (mean 70, SD 13.45) and post (mean 82, SD 12.12) forced vital capacity percent improved in all patients. A total of 41,181 skin cells were annotated into 10 major cell types and a total of 38,236 PBMCs were annotated into 5 major cell types from the sequencing data. 11,293 skin fibroblasts (FB) were sub-clustered into 7 FB subtypes, with COL11A1+, LTBP2+, and myofibroblasts (CILP_COL8A1+ FBs) showing diminished expression of the pro-fibrotic genes CTGF (CCN2), TAGLN, and VGLL3 post-transplantation at the levels comparable to healthy controls. Enriched cell-cell communication of the CD6/ALCAM pathway was noted only in pre-treatment skin biopsies but neither in post-treatment nor in controls. Additionally, integrin-mediated signaling pathway was found to be enriched in the fibroblasts of pre-treatment skin, and not in the post-treatment skin biopsies (Figure 1). In PBMCs, CD4 T cell proportion was similar in controls and pre-treatment samples but reduced in post-treatment biopsies. LCK pathway, involved in activation of T cell signaling, was found to be associated with cell-cell communication only in pre-treatment PBMC samples but absent in both post-treatment and healthy control.
Conclusion: Our data identify a reduction in expression of pro-fibrotic genes in FB subtypes and diminished signaling of pathways involved in mediating inflammation in SSc-ILD patients who underwent HSCT therapy.Cottin, V., Brown, K.K. Interstitial lung disease associated with systemic sclerosis (SSc-ILD). Respir Res 20, 13 (2019). https://doi.org/10.1186/s12931-019-0980-7
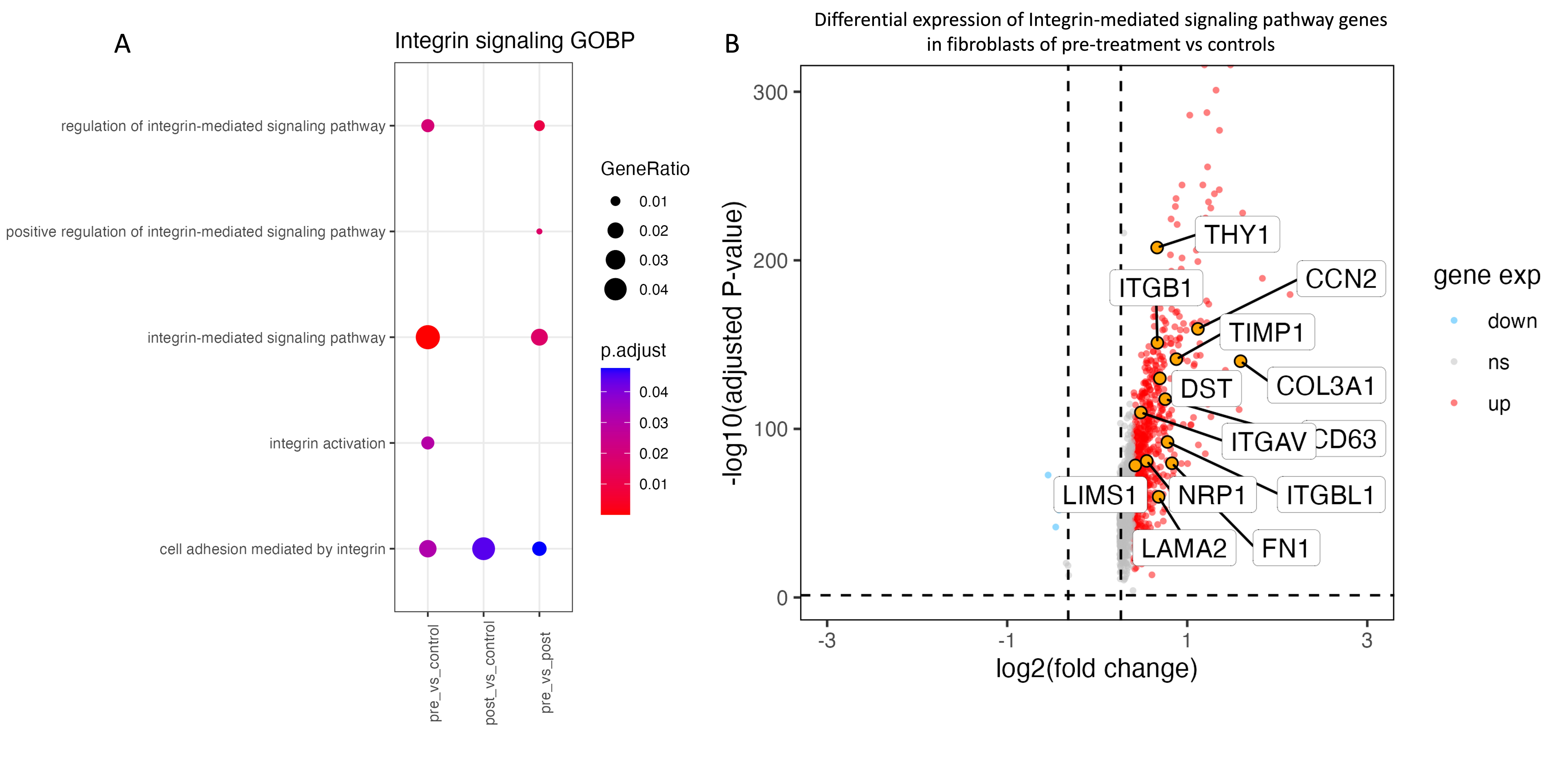 Figure 1: A. Integrin-mediated signaling pathway enriched in the fibroblasts of only pre-treatment skin (pre_vs_control), and not of the post-treatment skin biopsies (post_vs_control). B. Integrin-mediated signaling pathway genes up-regulated in fibroblasts of pre-treatment skin compared to the control skin
Figure 1: A. Integrin-mediated signaling pathway enriched in the fibroblasts of only pre-treatment skin (pre_vs_control), and not of the post-treatment skin biopsies (post_vs_control). B. Integrin-mediated signaling pathway genes up-regulated in fibroblasts of pre-treatment skin compared to the control skin
To cite this abstract in AMA style:
Dey P, Bogle R, Li Q, Plazyo O, Gedert R, Zahn C, Tsou P, Varga J, Tsoi L, Gudjonsson J, Khanna D. Effect of autologous myeloablative hematopoietic stem cell transplantation on skin and peripheral blood mononuclear cells in systemic sclerosis patients with interstitial lung disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effect-of-autologous-myeloablative-hematopoietic-stem-cell-transplantation-on-skin-and-peripheral-blood-mononuclear-cells-in-systemic-sclerosis-patients-with-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-autologous-myeloablative-hematopoietic-stem-cell-transplantation-on-skin-and-peripheral-blood-mononuclear-cells-in-systemic-sclerosis-patients-with-interstitial-lung-disease/
