Session Information
Date: Monday, November 14, 2022
Title: RA – Treatment Poster IV
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Rheumatoid arthritis (RA) is a chronic, autoimmune disease, which normally is characterized by inflammation and thickening of the synovial membrane leading to swollen and tender joints. Multiple phenotypes of RA have been suggested, including a fibrotic phenotype with limited inflammation (1). Such phenotype may respond differently to RA treatment, targeting inflammation. We hypothesize that a fibrotic component can be characterized by serological markers of the extracellular matrix and fibrogenesis. PRO-C3 is a measure of type III collagen formation and a known predictor of fibrogenesis in multiple fibro-inflammatory diseases including interstitial lung disease and fibrotic liver disease (2). The aim of this study was to measure PRO-C3 in two clinical studies testing the efficacy of the anti-IL6 receptor antagonist tocilizumab (TCZ) in RA patients with moderate to high disease activity (DAS28), to investigate the effect (or lack of effect) of TCZ in patients with measurable levels of fibrogenesis.
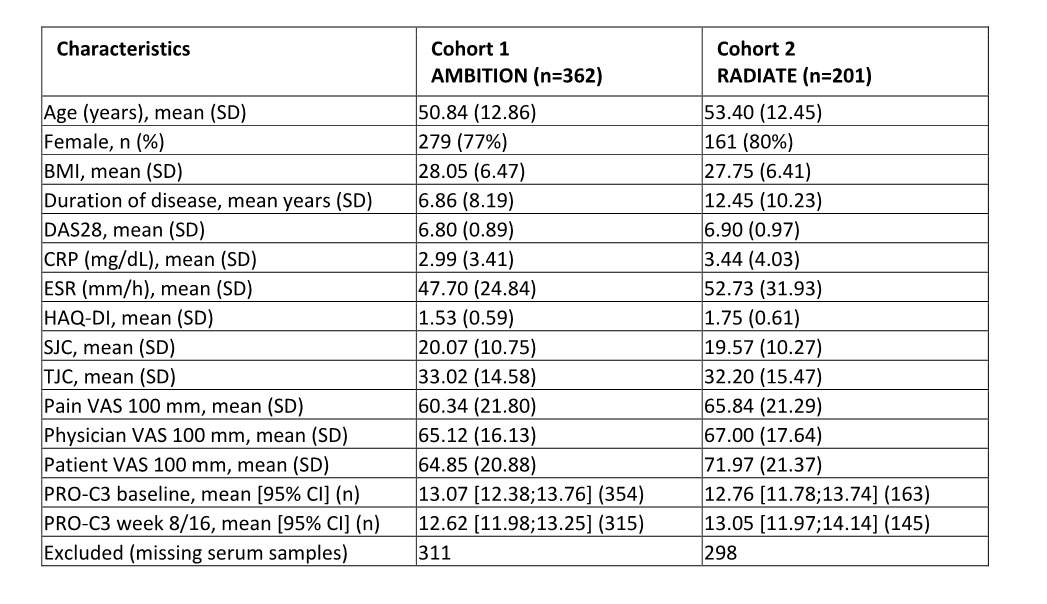
Methods: This study was a post-hoc exploratory analysis of biomarker cohorts derived from two independent clinical trials assessing the efficacy of TCZ as monotherapy or in combination with methotrexate (MTX), compared to MTX as monotherapy or placebo. PRO-C3 was measured in serum samples at baseline and follow-up (week 8 or 16), with a total of 563 RA patients available. The patients in cohort 1 had early-stage RA with no failed treatment and the patients in cohort 2 had late-stage RA with stable MTX treatment and inadequate response to anti-TNFα treatments. Duration of disease and ESR levels were the only clinically significant differences between the cohorts (p< 0.0001, table 1). PRO-C3 cut-offs were identified using DAS28 levels as outcome, responders defined as DAS28 ≤ 2.6 (remission) and non-responders as DAS28 > 2.6, and PRO-C3 levels as the predictor. Response rates and odds ratios (OR) were calculated using 2×2 contingency tables.
Results: The fibrogenesis biomarker, PRO-C3, did not correlate with any of the clinical measurements and no change in PRO-C3 was observed from baseline to follow-up in response to treatment. In the early-stage cohort, patients with high levels of PRO-C3 ( >9.5 ng/mL) had a response rate of 6.6% compared to 16.5% in the patients with low levels of PRO-C3 (OR = 0.34, 95% CI [0.18, 0.90], p=0.042). In the late-stage cohort, patients with high PRO-C3 levels ( >9.8 ng/mL) had a response rate of 8.6% compared to 18.8% in the patients with low levels of PRO-C3 (OR = 0.46, 95% CI [0.18, 1.28], p=0.18).
Conclusion: RA patients with high level of measurable fibrogenesis were less likely to respond to TCZ, as compared to patients with low level of fibrogenesis. These data indicate the existence of a fibrotic phenotype thus, patients presenting this phenotype may potentially benefit from anti-fibrotic treatments.
References:
1. Dennis G et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res Ther. 2014;16(2):R90.
2. Karsdal MA et al. Profiling and targeting connective tissue remodeling in autoimmunity – A novel paradigm for diagnosing and treating chronic diseases. Autoimmun Rev. 2021;20(1):102706.
BMI: Body mass index, DAS28: Disease activity score_28, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, HAQ-DI: Health Assessment Questionnaire-Disability Index, SJC: swollen joint count, TJC: tender joint count, VAS: visual analogue scale, PRO-C3: biomarker of type III collagen formation.
To cite this abstract in AMA style:
Falkenløve Madsen S, Sinkeviciute D, Thudium C, Karsdal M, Bay-Jensen A. Early-stage Rheumatoid Arthritis Patients with High Levels of Type III Collagen Are Less Likely to Respond to Anti-IL6R Treatment [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/early-stage-rheumatoid-arthritis-patients-with-high-levels-of-type-iii-collagen-are-less-likely-to-respond-to-anti-il6r-treatment/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-stage-rheumatoid-arthritis-patients-with-high-levels-of-type-iii-collagen-are-less-likely-to-respond-to-anti-il6r-treatment/