Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 8:45AM-9:00AM
Background/Purpose: Excessive formation and impaired clearance of Neutrophil Extracellular Traps (NETs) have been linked to autoimmune and inflammatory diseases, notably systemic lupus erythematosus (SLE). DNASE1-like 3 (DNASE1L3) is a circulating endonuclease that degrades NETs, and loss-of-function mutations are associated with severe autoimmune phenotypes in patients, including childhood-onset SLE, vasculitis, and systemic sclerosis. Based on this biology, Neutrolis developed a platform of engineered recombinant DNASE1L3 analogues, including NTR-441, a DNASE1L3 analogue fused to human serum albumin. In this first-in-human study, we aimed to validate the novel approach of NET inactivation by DNASE1L3 as a therapeutic strategy with three main objectives:
(1) assess safety, tolerability, and pharmacokinetics (PK) in healthy subject lacking the pharmacological target (NETs)
(2) evaluate safety, tolerability, PK, and target engagement in patients with a high NET burden
(3) establish pharmacodynamic and clinical proof-of-concept in a patient with SLE and congenital DNASE1L3 deficiency
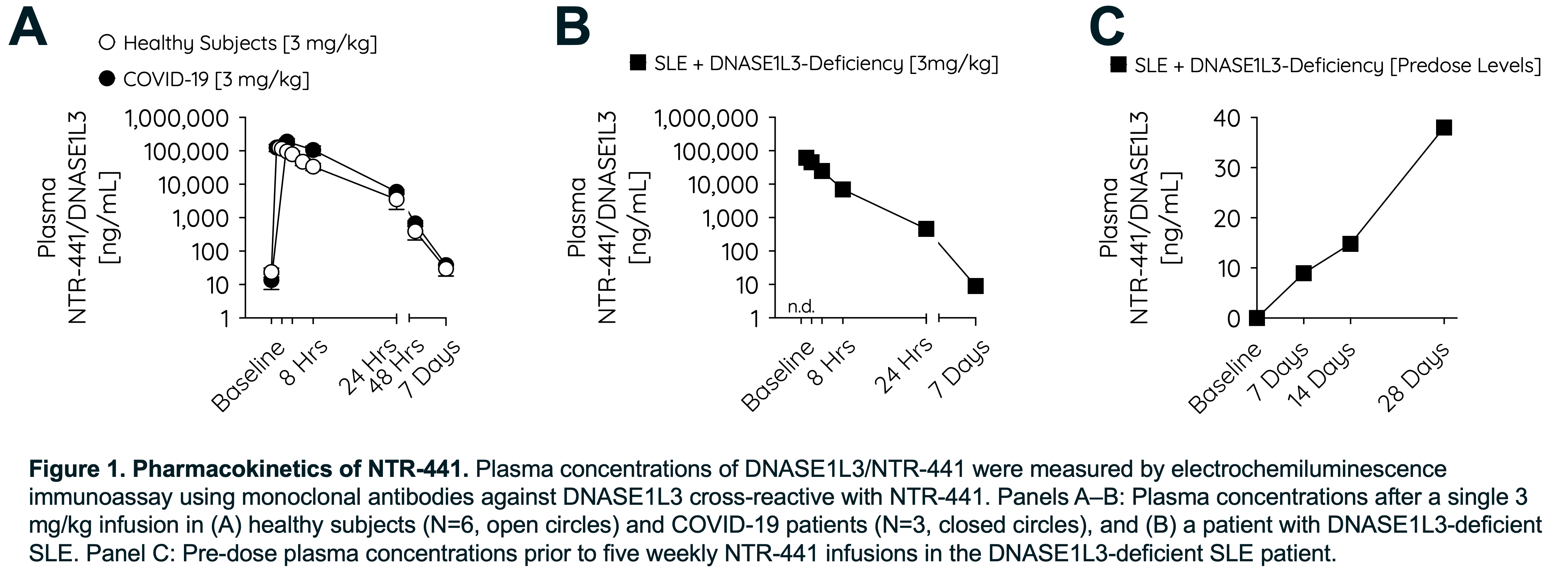
Methods: A first-in-human Phase 1a/b, placebo-controlled, double-blind trial was conducted, including a single ascending dose study in 35 healthy adults and 12 hospitalized patients with severe COVID-19, representing a high NET burden disease (NCT04941183) across dose levels of 0.01–10 mg/kg. Proof-of-concept was investigated in a 16.5-year-old male with a homozygous DNASE1L3 loss-of-function mutation (c.643del) and severe, longstanding, treatment-refractory monogenic SLE, who received weekly doses of NTR-441 (3 mg/kg) as add-on therapy to corticosteroids, antimalarials, and tofacitinib through a compassionate use protocol (Ethics Committee approval no. 0120-553/2024-2711-8).
Results: NTR-441 was well tolerated across all studies and produced robust increases in DNASE1L3 levels that remained supraphysiologic for >48 hours (Fig. 1). Infusion reactions occurred in one healthy subject at the highest dose and at the fifth dose in the patient with SLE, the latter linked to anti-drug antibodies but managed with desensitization and adjusted dosing. Target engagement was demonstrated in patients with COVID-19 and SLE by rises in NET-degradation products (MPO–DNA complexes, cell-free DNA) peaking 4–8 hours post-infusion, while absent in healthy volunteers (Fig. 2). In the SLE patient, the first dose of NTR-441 resulted in a rapid (≤6 hours) and sustained clinical improvement across multiple organ systems, including urticarial rash, episcleritis, and arthritis except for a transient rash recurrence that subsequently subsided (Fig. 3).
Conclusion: These combined studies validate DNASE1L3 as a therapeutic strategy to inactivate NETs. In a patient with treatment-refractory SLE with DNASE1L3-deficiency, pharmacologic degradation of NETs with NTR-441 induced rapid, sustained remission, providing the first clinical proof-of-concept for this approach. The temporal link between NET clearance and clinical benefit supports NETs as a central pathogenic driver in autoimmunity. These findings warrant broader trials in SLE and other autoimmune disorders.
To cite this abstract in AMA style:
Reiff A, Avcin T, Jilma B, Korosec P, Weiss-Tessbach M, Schoergenhofer C, Bizjak M, Jenko Bizjan B, Cugalj Kern B, Simpfendorfer K, Lood C, Artner T, Prasanna A, Olivier K, Gouya G, Lambalot R, Hakkim A, Fuchs T. Early Evidence of Proof-of-Concept of an Albumin-DNASE1L3 Fusion Protein (NTR-441) for the Rapid Enzymatic Inactivation of NETs in SLE with DNASE1L3-Deficiency [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/early-evidence-of-proof-of-concept-of-an-albumin-dnase1l3-fusion-protein-ntr-441-for-the-rapid-enzymatic-inactivation-of-nets-in-sle-with-dnase1l3-deficiency/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-evidence-of-proof-of-concept-of-an-albumin-dnase1l3-fusion-protein-ntr-441-for-the-rapid-enzymatic-inactivation-of-nets-in-sle-with-dnase1l3-deficiency/

.jpg)
.jpg)