Session Information
Date: Monday, November 13, 2023
Title: (1100–1123) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Dotinurad is a potent and selective URAT1 inhibitor that has been approved as a once-daily drug for the treatment of hyperuricemia with or without gout in Japan since 2020, based on results of 17 clinical trials conducted in Japan with more than 1000 patients exposed with up to 58 weeks in treatment duration. In the Japanese dossier, 4mg dotinurad reduced serum urate to < 6 mg/dL in 100% of patients after long-term treatment. No significant cardiovascular, renal or hepatic adverse events were observed with dotinurad at approved doses in the Japanese studies. This Phase 1 study is the first performed in a ‘western’ population, initiating the development of dotinurad for eventual use in North America and EU.
Methods: Randomized, double-blind, placebo-controlled, three-period crossover multiple-dose in 15 healthy male and female volunteers (age 18 to 55 years), doses included placebo, 1, 2, 4, and 8 mg once-daily in the morning for 4 days. Serial blood samples were collected to measure pharmacokinetic concentrations of dotinurad as well as serum and urine uric acid levels.Maximum concentration (Cmax), area under the curve (AUC0-τ), half-life (t1/2) were determined.Study drug accumulation from Day 1 to Day 4 was assessed. Safety monitoring was performed throughout.
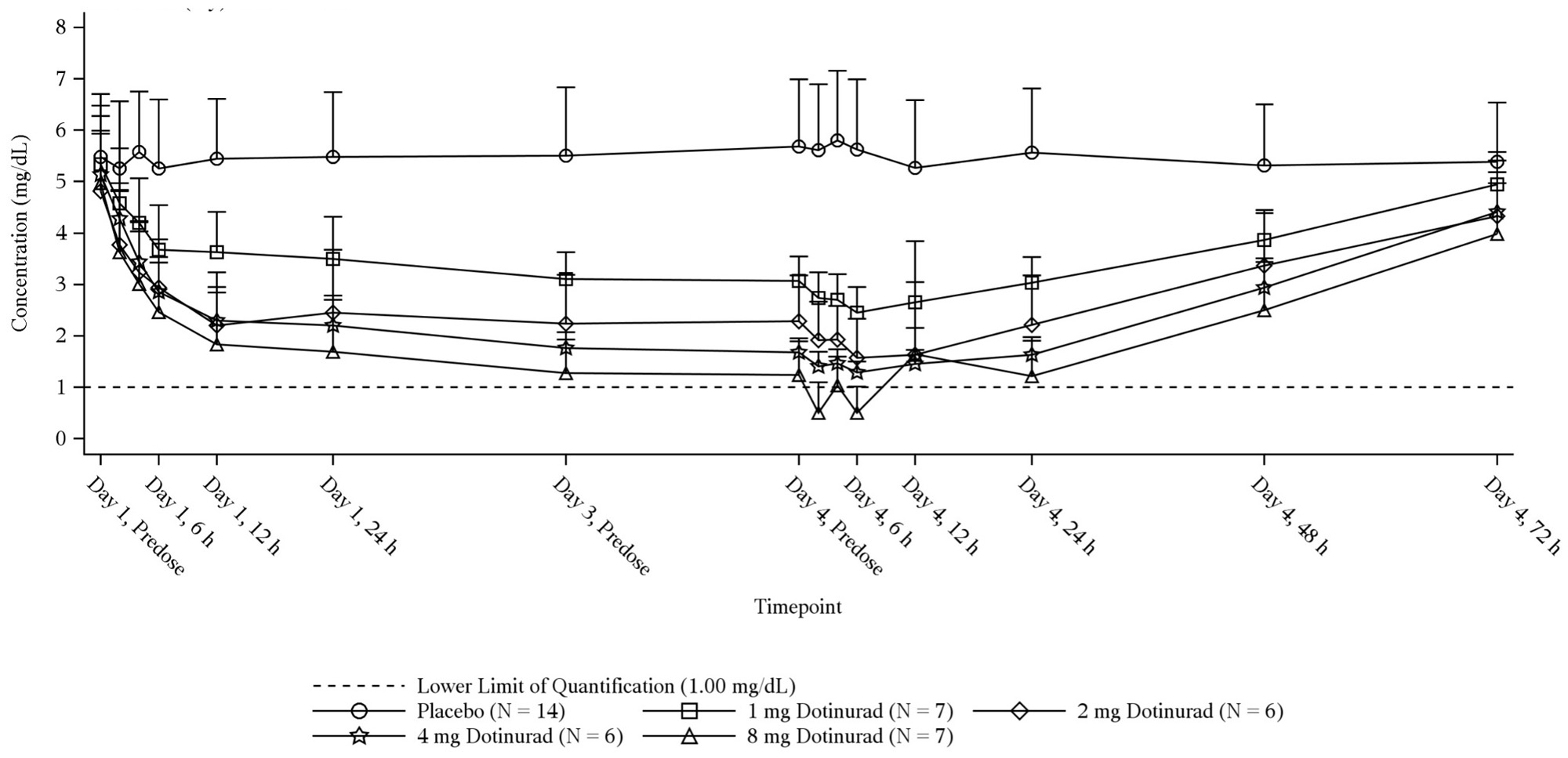
Results: Dotinurad concentrations exhibited an increase in dotinurad exposure for AUC and Cmax that was proportional to dose.Plasma-concentration time profiles following multiple oral doses of dotinurad were characterized by a rapid absorption phase, with median Tmax of 2.5 to 3 hours. When normalized for dose, Cmax was consistent across the dose range. The rate of elimination appeared to be consistent across the dose groups on Day 4, with geometric mean T1/2 values ranging from 7.73 to 8.82 hours. The range of accumulation ratios for AUC0-τ and Cmax were also found to be similar across all doses administered (1.18 to 1.26 and 1.07 to 1.21, respectively), demonstrating that dotinurad shows little to no accumulation in the plasma following multiple daily dosing.
As dotinurad dose increased, a respective reduction in serum urate concentrations and overall exposure to urate was observed. Steady state of reduced serum urate levels was achieved in day 3. Up to 90% sUA reduction from baseline was observed with dotinurad treatment.
Dotinurad was well tolerated with no observed serious adverse event. No treatment related adverse event was observed with 1-4 mg of dotinurad.
Conclusion: Following 4 consecutive once-daily treatments, dotinurad has exhibited a pharmacokinetic profile, in western healthy subjects, comparable to that from Japanese healthy men. All tested doses of dotinurad (1, 2, 4 & 8 mg QD) significantly reduced serum urate levels. These results support the potential application of dotinurad as a once-daily treatment for hyperuricemia in western population. Another clinical study is currently underway to further establish the safety, efficacy and drug interaction of dotinurad in U.S. gout patients.
To cite this abstract in AMA style:
Baumgartner S, Zheng R, Harnett M, Kranzler J. Dotinurad, a Potent and Selective Uricosuric Agent, Exhibited Promising Pharmacokinetics and Pharmacodynamic Profiles to Significantly Reduce Serum Urate Levels Following Once Daily Dosing in Healthy U.S. Subjects in a Phase 1 Clinical Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/dotinurad-a-potent-and-selective-uricosuric-agent-exhibited-promising-pharmacokinetics-and-pharmacodynamic-profiles-to-significantly-reduce-serum-urate-levels-following-once-daily-dosing-in-healthy/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dotinurad-a-potent-and-selective-uricosuric-agent-exhibited-promising-pharmacokinetics-and-pharmacodynamic-profiles-to-significantly-reduce-serum-urate-levels-following-once-daily-dosing-in-healthy/