Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Ianalumab (VAY736) is an afuscosylated monoclonal antibody targeting the B cell activating factor receptor (BAFFR) that depletes B cells via enhanced antibody dependent cellular cytotoxicity with concurrent blockade of BAFF:BAFFR mediated survival signals. Sjögren’s disease (SjD) is an autoimmune disorder which primarily affects exocrine glands along with diverse systemic manifestations. It is characterized by elevated BAFF levels, hypergammaglobulinemia and autoantibodies to nuclear autoantigens. A phase 2b dose finding trial of ianalumab (NCT02962895) in 191 patients with active SjD met its primary endpoint, identifying 300 mg as the most clinically efficacious dose, despite a similar reduction in blood B cells as seen with 50 mg. We explored the changes in autoantibody and proteomic signatures of patients with SjD pre- and post-treatment with ianalumab to characterize pharmacodynamic and biological biomarkers associated with disease activity and clinical response.
Methods: Patients with active SjD (N=191) were randomly assigned 1:1:1:1 to receive placebo or one of three different doses of ianalumab (5 mg, 50 mg, or 300 mg). Serum samples were collected at baseline and Week 24. Serum protein profiling was performed using the SomaScan(R) v4.1 platform measuring >7000 aptamers. The interferon protein signature (IFNPS) was derived from the SomaScan data. Autoantibodies and BAFF levels were assessed by Luminex-based and ELISA assays. A linear mixed effect model was used to identify longitudinal changes in protein concentration at Week 24 versus baseline, and proteins with FDR < 0.05 and log fc >0.1 were selected for further analysis. Results were visualized using heatmaps and hierarchical clustering based on protein expression patterns.
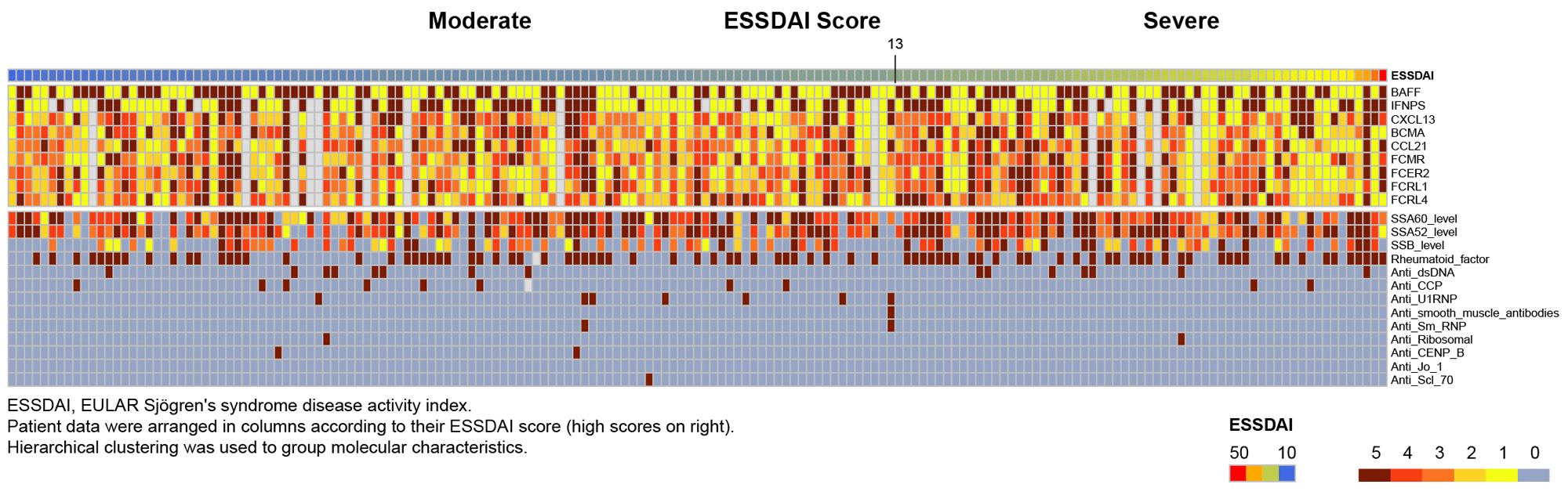
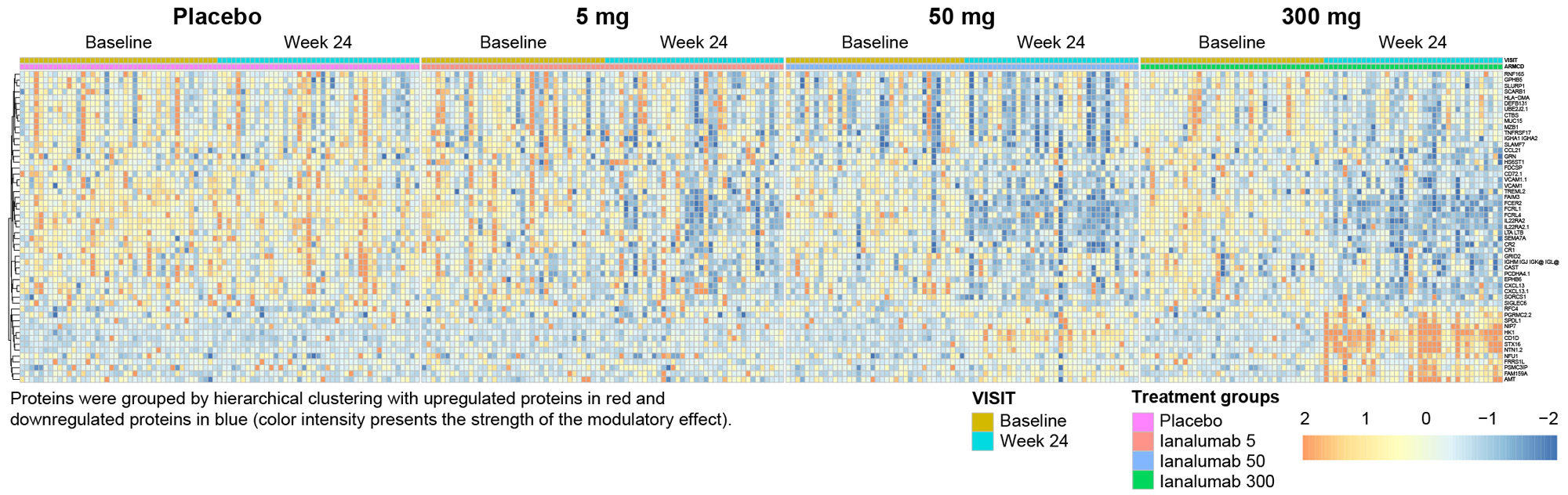
Results: A cluster analysis performed on autoantibodies and proteins, including IFNPS at baseline did not reveal significant correlations between ESSDAI scores (including subdomains) and levels of autoantibodies or proteins of interest (Fig 1).Administration of ianalumab led to marked changes in autoantibody and serum protein levels. The 300 mg dose group showed an increased number of significantly modulated serum proteins (42), versus the 5 mg (9) and 50 mg (20) dose groups, along with a more pronounced modulation of their expression (Fig 2). Several B cell surface proteins were consistently downregulated by ianalumab, including FCRL4, expressed by B cells hypothesized to be involved in the pathogenicity of SjD. The 50 mg and 300 mg doses induced the downregulation of additional proteins such as BCMA, specifically expressed by antibody producing cells, or the chemokines CXCL13 and CCL21, associated with immune infiltration of glandular tissues in patients with SjD. Although statistically non-significant, a downregulation of IFNPS was also seen with 50 mg and 300 mg doses.
Conclusion: No differential proteomic signatures related to baseline disease activity were identified. The dose response in clinical efficacy of ianalumab in treating patients with SjD as observed in the phase 2b trial is reflected at the protein level, with increased depth and breadth of proteomic changes by Week 24 correlating with increasing dose.
To cite this abstract in AMA style:
Finzel S, Grioni A, A Fisher B, S Papas A, Avrameas A, Tuckwell D, Zierer J, Rauld C, De Luca V, Ferrero E, Nogueira da Costa A, Hillenbrand R, Isnardi I, Hueber W. Dose Dependent Modulation of a B Cell Protein Signature by Ianalumab in Patients with Sjögren’s Disease [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/dose-dependent-modulation-of-a-b-cell-protein-signature-by-ianalumab-in-patients-with-sjogrens-disease/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dose-dependent-modulation-of-a-b-cell-protein-signature-by-ianalumab-in-patients-with-sjogrens-disease/